Keywords
drug resistance, Plasmodium falciparum, antifolate, artemisinin, genetic markers, Eritrea
This article is included in the Pathogens gateway.
drug resistance, Plasmodium falciparum, antifolate, artemisinin, genetic markers, Eritrea
Paper changes for: Screening for antifolate and artemisinin resistance in Plasmodium falciparum clinical isolates from three hospitals of Eritrea
Sample characteristics
A statement was added at the end of the paragraph describing the isolate serial number
PCR amplification and point mutation analyses
The number of Pfdhps substitutions analyzed was changed from one to two – this has been described as Pfdhps substitution at position 1347 translating as a synonymous mutation (R449R) at the amino acid level.
The change described above in (a) was also added in the caption for Figure 1
Changes were made in Table 4 under Pfdhps and PfK-13 respectively clearly indicating the corresponding nucleotide- (depicted in a yellow highlight ) and amino acid base changes analyzed in the study.
See the authors' detailed response to the review by Sam L Nsobya
See the authors' detailed response to the review by Olusola Ojurongbe
Malaria is a major vector-borne disease, endemic in 87 tropical and sub-tropical countries, causing over 400,000 deaths yearly (WHO World Malaria Report 2020). Eritrea, which is situated in the Horn of Africa, has experienced a significant decline in deaths and cases of malaria over the past 20 years (WHO World Malaria Report 2019). This reduction, according to the Ministry of Health (MOH) reports, is mainly due to extensive interventions employed towards the control of malaria since the establishment of the Eritrea National Malaria Control Program (NMCP) in 1995.1 Working hand-in-hand with Roll Back Malaria (RBM) collaborators and stakeholders,2 NMCP set up a combination of strategies including integrated vector management (IVM), early diagnosis and prompt treatment3 consequently leading to a remarkable decrease in incidence and mortality rates, following the gruesome 1998 malaria epidemic in the country.4 The disease is generally endemic in the Western lowlands of Gash Barka, Anseba, Debub and Semenawi Keih Bahri (Northern Red Sea) zobas (regions) whereas the Central highlands and Eastern lowlands of Maekel and Debubawi Keih Bahri (Southern Red Sea) zobas respectively have unstable, seasonal transmission. July–September is the common rainy season and hence malaria transmission peaks between October–November in a majority of the endemic areas while in the Coastal region the rainy season mostly occurs between December–January leading to a heightened transmission in March–April.5,6 About three-quarters of confirmed malaria cases in Eritrea are caused by Plasmodium falciparum and the remaining one-quarter is attributed to Plasmodium vivax, as well as small proportions of mixed infections (WHO African Region: Eritrea 2018). Currently, case management in Eritrea exclusively entails World Health Organisation (WHO) recommended first line treatment of uncomplicated malaria using artesunate-amodiaquine (AS-AQ), an artemisinin-based combination therapy (ACT) adopted in 2007, while quinine (Q) has been used for severe cases of infection since 2002 (WHO African Region: Eritrea 2018). Monitoring for drug resistance plays a major role in governing the efficacy of antimalarials, which subsequently influences their use in a population.
The emergence of drug resistance, especially among P. falciparum parasites, is a major hindrance to malaria control due to its increasing prevalence to essentially all antimalarials including sulfadoxine-pyrimethamine (SP) and lately artemisinins (ARTs).7 Genetic markers are invaluable tools in screening and detection of drug resistance, in addition to predicting the efficacy of antimalarials.8 Sulfadoxine-pyrimethamine P. falciparum resistance (SPR), which is well-studied, results from the occurrence and accumulation of mutations in the dihydrofolate reductase gene (Pfdhfr) and in the dihydropteorate synthase gene (Pfdhps) leading to a gradual reduction of sensitivity to pyrimethamine and sulfadoxine respectively.9 In vitro and in vivo studies have shown that SPR is mainly associated with point mutations at codons N51I, C59R, S108N and I164L of Pfdhfr and S436A, A437G, K540E, A581G and A613S of Pfdhps.10,11 Various combinations of these mutations have been used to classify SP resistant parasites according to different levels of resistance i.e. partially-, fully- or super resistant parasites and this has subsequently affected SP treatment policy. Partial resistance is demonstrated by a combination of triple mutant Pfdhfr, N51I, C59R, S108N and Pfdhps, A437G whereas full resistance is shown by a combination of triple mutant Pfdhfr, N51I, C59R, S108N and double mutant Pfdhfr, A437G, K540E. Finally, the sextuple mutant genotype involving a combination of triple mutant Pfdhfr, N51I, C59R, S108N and triple mutant Pdhfr, A437G, K540E and A581G defines super resistance.12
The development of artemisinin (ART) resistant P. falciparum parasites was first independently described in Western Cambodia, South East Asia.13 To date, resistance is commonly associated with five non-synonymous mutations including M476I, Y493H, R539T, I543T, and C580Y in the propeller domain of P. falciparum kelch 13 gene (Pfk13).14,15 ART resistance is primarily characterized by delayed parasite clearance rates in clinical studies as well as reduced in vitro drug susceptibility of the ring stage of parasite development.16,17 Considering the significant malaria control interventions accomplished in Eritrea, this pilot study aimed at availing supplementary molecular data by screening for SP and ART resistance-associated mutations from a cohort of patients, treated with first line AS-AQ, visiting selected hospitals located in malaria endemic regions of Eritrea. Generally, despite WHO’s change in treatment policy from the chloroquine (CQ) - sulfadoxine-pyrimethamine (SP) combination, adopted in 2002 to ACT, little is documented on the genetic mechanism underlying SPR using genetic markers. Additionally, a continuous detection for ART-resistance using genetic markers is important to keep track of changes at the genetic level.
The ethical approval for this study was obtained from the Eritrea Institute of Technology, Research and Postgraduate Studies (RPS) Ethics Review Committee (Reference no. RPS/169/14) and the Ethics Review Board of the National Commission for Higher Education, Eritrea (NCHE) (Reference no. BHEAIL/3/656-568/14).
Sample collection was conducted from 1st July to 1st October 2014 at three hospitals located in malaria-endemic zobas of Eritrea: Adi Quala Hospital, Adi Quala (14°38′07′′N, 38°50′03′′E) in Zoba Debub, Keren Hospital, Keren (15°46′40′′N, 38°27′03′′E) in Zoba Anseba and Gash Barka Referral Hospital, Barentu (15°06′20′′N, 37°35′26′′E) in Zoba Gash Barka. Three time ranges were employed for the study at the three hospitals: from 1st July to 31st August 2014 for Adi Qualla Hospital, 16th July to 15th September 2014 for Keren Hospital and 15th August to 1st October 2014 for Gash Barka Referral Hospital.
Blood samples were obtained from patients with febrile illness, who visited the three hospitals within the study period. The samples were spotted on Whatman 903TM paper (GE Healthcare Bioscience Corp.), stored in individual plastic bags with silica desiccant and transported for further molecular studies at the Institute for Biotechnology Research (IBR) in Jomo Kenyatta University of Agriculture and Technology (JKUAT), Kenya.
Genomic DNA extraction was performed on the dried blood spot (DBS) samples using Schneeberger’s protocol with slight modifications, comprising 1.5M guanidine thiocyanate and 100mM Tris with 0.1% sodium dodecyl sulfate (SDS) at pH 8.18 Concentration of DNA ranged from 0.05 ng/uL to 6.03 ng/uL whereas the ratio obtained from analysis of DNA purity (260 nm/280 nm) ranged from 1.4 to 2.17. The DNA extracts were stored at -20°C and used for PCR amplification.
Outer and nested PCR amplification was conducted using the AB 9800 Fast Thermocycler machine (Applied Biosystems, UK) on regions flanking identified point mutations of the following P. falciparum genes: bifunctional dihydrofolate reductase-thymidylate synthase – DHFR-TS (PF3D7_0417200), i.e. N51I, C59R, and S108N, hydroxymethyldihydropterin pyrophosphokinase-dihydropteroate synthase – PPPK-DHPS (PF3D7_0810800) i.e. K540E and kelch protein – kelch 13 (PF3D7_1343700) i.e. Y493H, R539T, I543T, and C580Y which confer drug resistance to SP and ART respectively. The respective gene sequences were retrieved from PlasmoDB release 46 (http://PlasmoDB.org) and primer design (Table 1) was performed using PrimerQuest and OligoAnalyzer tools from Integrated DNA technologies online platform (https://www.idtdna.com/). Selection of primers considered characteristics such as: Guanine-Cytosine (G+C) content of greater than 50, five degrees difference between melting temperatures and absence of hair-pin formation and self-annealing properties. A total PCR volume of 25 uL containing 12.5 uL of 2× DreamTaq PCR master mix (Thermo ScientificTM), 3.75 uL of the DNA template and 0.25 uL of the forward and reverse primers respectively were obtained for all the reactions. A volume of 3.75 uL of DNA template in the outer primary PCR reaction, as well as for the PCR amplicon in the nested secondary reaction was used. Step-down PCR cycling conditions for the outer and nested reactions were set as follows: an initial denaturation of 94°C for three minutes, a denaturation of 94°C for 15 seconds, an annealing temperature range of 55°C–60°C for 30 seconds, an elongation of 72°C for one minute and a final elongation of 72°C for 10 minutes.
Resolution of PCR amplicons was run in 1.5% agarose gel, 1× TAE buffer, at 70 V, 58 mA for one hour 30 minutes using a gel electrophoresis system (IBI-Shelton Scientific MP-1015 multipurpose) and an electrophoresis power voltage supplier (Pharmacia LKB ECPS 3000V/150mA). GelRed ® Nucleic Acid Gel stain (Biotium) was used for pre-cast gel staining, 1 kb DNA ladder (Thermo ScientificTM) for DNA quantification of resolved PCR amplicons. P. falciparum 3D7 purified DNA laboratory strain obtained from Kenya Medical Research Institute (KEMRI) was used as the main control for wild-type and mutant alleles of each gene. Purification of nested PCR amplicons depicting a single band was performed using the QIAquick PCR purification kit (Qiagen) whereas for amplicons showing double bands, the targets were processed using QIAquick gel extraction kit (Qiagen) as per the manufacturer’s protocol respectively. The PCR amplicons were shipped to Macrogen (Seoul, Korea) for Sanger sequencing.
QIAGEN CLC Main Workbench v21.0.4 was used to perform sequence data editing, consensus sequence assembly and identification of nucleotide base conflicts against the 3D7 reference gene sequences of PF3D7_0417200, PF3D7_0810800 and PF3D7_1343700. Multiple sequence alignment (MSA), was carried out in MEGA v7.019 using the Muscle algorithm20 to identify nucleotide base changes, including translation to amino acid sequences using the standard genetic code for the identification of amino acid changes and their respective positions. Further visualisation of sequence alignments was performed in Jalview v2.11.1.421 to identify non-synonymous point mutations.
After consent was given for participation and follow up, 19 dried blood spot (DBS) samples were successfully collected from a total of 131 patients who visited the three hospitals during the study period,22 10 samples from Adi Quala Hospital (AQH = 10), three samples from Keren Hospital (KH = 3) and six samples from Gash Barka Referral Hospital (GBH = 6). Eight DBS samples were from patients treated with ACT (artesunate [AS] 100 mg + amodiaquine [AQ] 200 mg) and did not respond to treatment. These underwent re-treatment with quinine (Q) and were cured. Five DBS samples were from patients who responded to ACT treatment. The remaining six were from patients presenting severe illness and were treated with quinine (Q) (Table 2). The isolate serial number is depicted as the hospital code preceding a unique identifier i.e. AQHxxx, KHxxx, GBHxxx.
AS = artesunate, AQ = amodiaquine, Q = quinine.
On PCR amplification of targeted gene regions, sequence data from eight samples (AQH = 2, KH = 2, GBH = 4) was eventually analyzed for point mutations (Table 3). The nucleotide base changes comprised of four Pfdhfr substitutions, adenine (A) to cytosine (C) at position 152, thymine (T) to cytosine (C) at position 175, guanine (G) to adenine (A) at position 323, thymine (T) to cytosine (C) at position 134; two Pfdhps substitutions, adenine (A) to guanine (G) at position 1618 and 1347 and none identified for PfK-13 (Table 4). Subsequent translation to amino acid sequences constituted changes as follows: asparagine (N) to isoleucine (I) at codon 51, cysteine (C) to arginine (R) at codon 59, serine (S) to asparagine (N) at codon 108 and valine (V) to alanine (A) at codon 45 for Pfdhfr; lysine (K) to glutamate (E) at codon 540 and arginine (R) retained at codon 449 for Pfdhps and wild-type amino acids retained for Pfkelch-13 (Table 4). Multiple sequence alignment (MSA) and visualization of consensus sequence assemblies for Pfdhfr, Pfdhps and Pfkelch-13 against their 3D7 reference sequences distinguished four non-synonymous (nsy) point mutations for Pfdhfr (N51I, C59R, S108N, V45A), one non-synonymous (nsy) point mutation (K540E) and one synonymous (sy) point mutation (R449R) for Pfdhps while Pfkelch-13 retained wild-type amino acids (Figure 1).
N = asparagine, I = isoleucine, C = cysteine, R = arginine, S = serine, V = valine, A = alanine.
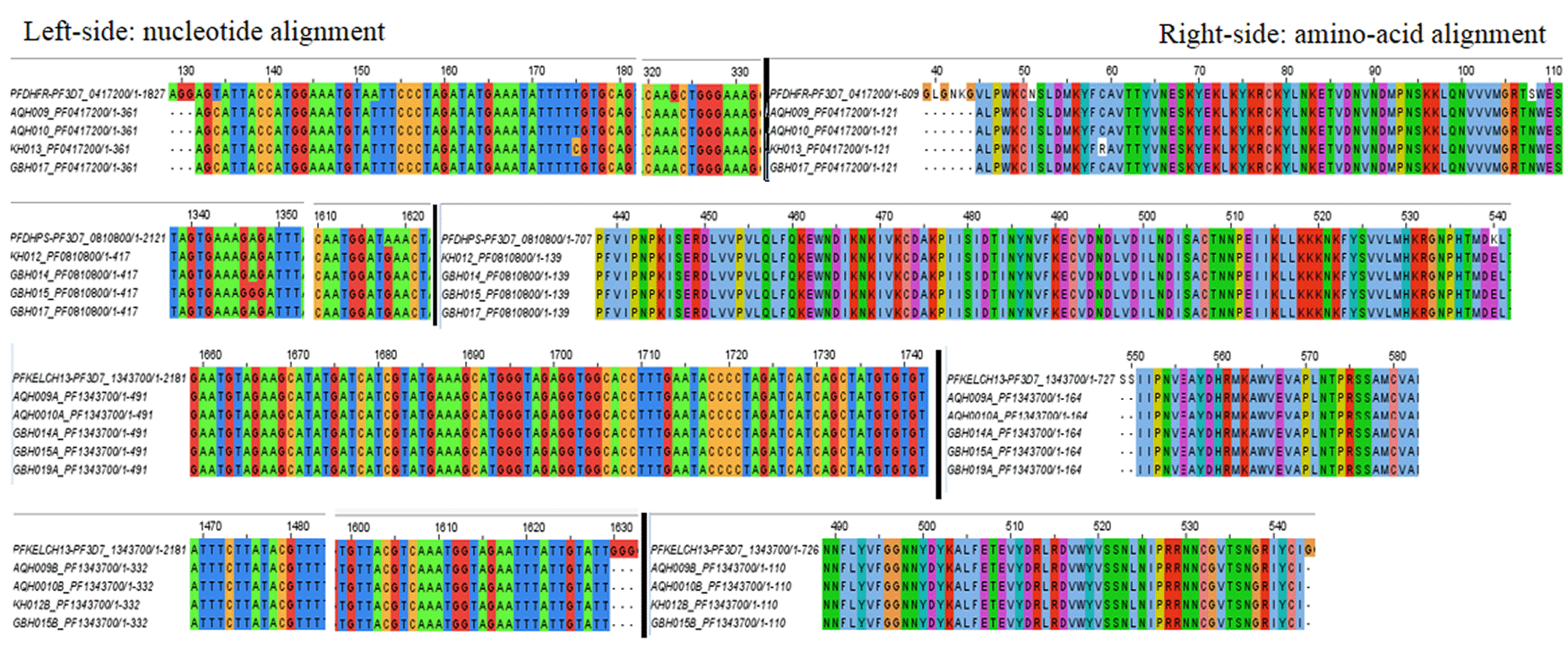
Jalview visualization of multiple sequence alignments depicting nsy- and sy-point mutations: Pfdhfr (N51I, S108N, V45A) occurred in all four isolates (KH013, GBH017, AQH010, AQH009), C59R was identified in one isolate (KH013); Pfdhps (K540E) occurred in all four isolates (KH013, GBH017, AQH010, AQH009), R449R was identified in one isolate (GBH015) and Pfdhps (K540E) occurred in all four isolates (KH012, GBH014, GBH015, GBH017) PfK-13, established no point mutations in all six isolates, wild type amino acids retained at c.554(S), c.566(V), c.569(A), c.578(A), c.580(C), c.493(Y), c.539(R), c.543 (I).
In this study, we present findings from a pilot survey assessing the occurrence of point mutations in PfK-13, Pfdhfr and Pfdhps genes from clinical isolates obtained from three zobas of Eritrea: Adi Quala (Adi Quala Hospital), Debub (Keren Hospital) and Anseba (Gash Barka Hospital). We targeted PCR-amplification of PfK-13 point mutations associated with artemisinin (ART) resistant phenotype in western Cambodia, South-East Asia Y493H, R539T, I543T, C580Y,14 non-synonymous point mutations, V566I, A578S, identified in isolates from five Sub-Saharan countries23 and N554S, A569S reported in a previous study from islands in Lake Victoria, Kenya.24 This study found none of the corresponding point mutations in PfK-13, which is similar to other studies from Eritrea25 and Kenya26,27 including other malaria endemic sub-Saharan countries.28 Data from the treatment outcome with the prescribed artemisinin (artesunate [AS]) indicated susceptibility responses corresponding with our findings and suggesting a likely absence of ART resistance. Only one isolate from these analyses was obtained from a patient who did not respond to first line treatment with AS-AQ – this could be attributed to possible causes of treatment failure such as non-compliance to the treatment regimen, incorrect drug usage, drug pharmacokinetics as well as host immunity.29,30
Pfdhfr point mutations, N51I, C59R and S108N observed in our study correspond with previous reports from Senegal,31 South Africa,32 Malawi, Mali, Kenya, Tanzania,33,34 including Venezuela in South America.35 Additionally, the single Pfdhfr C59R and Pfdhps K540E point mutations seen in our findings, have been shown to predict the occurrence of the Pfdhfr-Pfdhps quintuple mutant haplotype (Pfdhfr 51I/59R/108N + Pfdhps 437G/540E),36 which is associated with fully resistant SP parasites12 as well as SP treatment failure.37 The selection of these Pfdhfr-Pfdhps mutations from our findings, is attributable to the prior use of the CQ-SP combination as first-line treatment for clinical management of febrile disease especially at the primary health care level in Eritrea. Additionally, prior evidence shows that SP resistant parasites originated from South East Asia and consecutively spread into Sub-Saharan Africa,38,39 which eventually reached Eritrea too, as demonstrated in these findings. The valine (V) to alanine (A) change at codon 45 in Pfdhfr from this study, has not been previously reported, although, a converse occurrence of alanine (A) to valine (V) at codon 16 has been associated, both singly and doubly in combination to S108N mutation, with resistance to another antifolate, cycloguanil.40,41 Further investigation with a larger sample size is recommended to validate the selection of the V45A mutation in the population, as well as understand its implications to protein function in association with other established Pfdhfr mutations. Additionally, further detection of other SP resistance associated mutations not reported herein is recommended.
Although this study design availed treatment outcome information to compare with corresponding generated molecular data, limitation of sample size as well as DNA quality and quantity constrained further detection of PfK13 mutations associated with artemisinin resistance. Nonetheless, the general findings reported here, are not affected by these limitations and essentially provides useful molecular inference for further investigations.
Here, we provide molecular data verifying the genetic mechanism underlying SP resistance from selected participants of three regions of Eritrea. Pfdhfr C59R and Pfdhps K540E are reliable markers for the quintuple mutant haplotype conferring full resistance to SP. This study provides the molecular status of SP resistance in Eritrea. Continued monitoring of artemisinin resistance is recommended. Future studies should be carried out on a larger sample size since this study was a pilot survey involving a small sample size.
This project contains the following underlying data:
NCBI Gene: bifunctional dihydrofolate reductase-thymidylate synthase (DHFR-TS) [Plasmodium falciparum (malaria parasite)] Accession number MZ322415, https://www.ncbi.nlm.nih.gov/nuccore/MZ322415.
NCBI Gene: bifunctional dihydrofolate reductase-thymidylate synthase (DHFR-TS) [Plasmodium falciparum (malaria parasite)] Accession number MZ322416, https://www.ncbi.nlm.nih.gov/nuccore/MZ322416.
NCBI Gene: bifunctional dihydrofolate reductase-thymidylate synthase (DHFR-TS) [Plasmodium falciparum (malaria parasite)] Accession number MZ322417, https://www.ncbi.nlm.nih.gov/nuccore/MZ322417.
NCBI Gene: bifunctional dihydrofolate reductase-thymidylate synthase (DHFR-TS) [Plasmodium falciparum (malaria parasite)] Accession number MZ322418, https://www.ncbi.nlm.nih.gov/nuccore/MZ322418.
NCBI Gene: hydroxymethyldihydropterin pyrophosphokinase-dihydropteroate synthase (PPPK-DHPS) [Plasmodium falciparum (malaria parasite)] Accession number MZ322419, https://www.ncbi.nlm.nih.gov/nuccore/MZ322419.
NCBI Gene: hydroxymethyldihydropterin pyrophosphokinase-dihydropteroate synthase (PPPK-DHPS) [Plasmodium falciparum (malaria parasite)] Accession number MZ322420, https://www.ncbi.nlm.nih.gov/nuccore/MZ322420.
NCBI Gene: hydroxymethyldihydropterin pyrophosphokinase-dihydropteroate synthase (PPPK-DHPS) [Plasmodium falciparum (malaria parasite)] Accession number MZ322421, https://www.ncbi.nlm.nih.gov/nuccore/MZ322421.
NCBI Gene: hydroxymethyldihydropterin pyrophosphokinase-dihydropteroate synthase (PPPK-DHPS) [Plasmodium falciparum (malaria parasite)] Accession number MZ322422, https://www.ncbi.nlm.nih.gov/nuccore/MZ322422.
NCBI Gene: kelch protein (K13) (Kelch13) [Plasmodium falciparum (malaria parasite)] Accession number MZ322423, https://www.ncbi.nlm.nih.gov/nuccore/MZ322423.
NCBI Gene: kelch protein (K13) (Kelch13) [Plasmodium falciparum (malaria parasite)] Accession number MZ322424, https://www.ncbi.nlm.nih.gov/nuccore/MZ322424.
NCBI Gene: kelch protein (K13) (Kelch13) [Plasmodium falciparum (malaria parasite)] Accession number MZ322425, https://www.ncbi.nlm.nih.gov/nuccore/MZ322425.
NCBI Gene: kelch protein (K13) (Kelch13) [Plasmodium falciparum (malaria parasite)] Accession number MZ322426, https://www.ncbi.nlm.nih.gov/nuccore/MZ322426.
NCBI Gene: kelch protein (K13) (Kelch13) [Plasmodium falciparum (malaria parasite)] Accession number MZ322427, https://www.ncbi.nlm.nih.gov/nuccore/MZ322427.
NCBI Gene: kelch protein (K13) (Kelch13) [Plasmodium falciparum (malaria parasite)] Accession number MZ322428, https://www.ncbi.nlm.nih.gov/nuccore/MZ322428.
NCBI Gene: kelch protein (K13) (Kelch13) [Plasmodium falciparum (malaria parasite)] Accession number MZ322429, https://www.ncbi.nlm.nih.gov/nuccore/MZ322429.
NCBI Gene: kelch protein (K13) (Kelch13) [Plasmodium falciparum (malaria parasite)] Accession number MZ322430, https://www.ncbi.nlm.nih.gov/nuccore/MZ322430.
NCBI Gene: kelch protein (K13) (Kelch13) [Plasmodium falciparum (malaria parasite)] Accession number MZ322431, https://www.ncbi.nlm.nih.gov/nuccore/MZ322431.
Dryad: Extended data for ‘Screening for Antifolate and Artemisinin resistance in Plasmodium falciparum clinical isolates from three hospitals of Eritrea’, https://doi.org/10.5061/dryad.sbcc2fr6q.22
This project contains the following extended data:
• the total number of patients grouped according to age, who visited the three hospitals during the study period.
• gel images of Pfdhfr, Pfdhps and PfK13 genetic markers.
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
All participants were informed concerning the aim of the study, assent and written informed consent was given by patients, voluntary participation was allowed, and confidentiality of information collected ensured.
The authors are grateful to all the participants of the study from the three hospitals and to Mr. Moses Ogugo (International Livestock Research Institute – Kenya) for the technical support.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
No
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: PhD holder in malaria drug resistance expert for the past 27 years
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medical Parasitology
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Medical Parasitology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 4 (revision) 12 Jun 24 |
||
|
Version 3 (revision) 02 May 24 |
read | read |
|
Version 2 (revision) 02 Feb 23 |
read | read |
|
Version 1 21 Jul 21 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)