Keywords
Circadian rhythm, Bmal1, Photoreceptors, 661W cells, Oxidative stress, Antioxidant ,Nrf2, GPX
This article is included in the Cell & Molecular Biology gateway.
This article is included in the Eye Health gateway.
This article is included in the Circadian Clocks in Health and Disease collection.
Circadian rhythm, Bmal1, Photoreceptors, 661W cells, Oxidative stress, Antioxidant ,Nrf2, GPX
The presence of a retinal circadian clock in mammals was demonstrated in the 1990s,1,2 and many studies have shown that retinal circadian clocks control many physiological functions within the retinal tissue.3 Additional studies using mice in which clock genes have been deleted have reported that the disruption of retinal circadian clocks has a profound effect on the retina.4–7 In the retina, clock genes are expressed in the photoreceptors and inner nuclear and ganglion cell layers.8–11 In the photoreceptor layer, only the cone photoreceptors seem to express clock genes.9 Consistent with these observations, a series of recent studies using a retina-specific Bmal1 knockout (KO; Chx10Cre; Bmal1fl/fl) mouse have reported that removal of the Bmal1 gene abolished the circadian rhythm of the photic (cone) electroretinogram,4 altered the spectral identity,12 and the viability of the cone photoreceptors during aging.13 Hence, disruption of the circadian clock in the cones affected many biological functions of these cells.14
Photoreceptors, especially cones, have a high metabolic rate15,16 and contain more mitochondria than rods.17,18 Consequentially, cones produce a high level of reactive oxygen species (ROS). ROS are by-products of mitochondrial aerobic metabolism and an accumulation of ROS during aging is natural and inevitable.19,20 Elevated intracellular levels of ROS cause oxidation and damage to lipids, proteins, nucleotides, and mitochondria.21,22 The daily activity of an organism is closely connected with ROS production, and rhythmic ROS production/cellular oxidation is reported in many organs.23–25 Furthermore, the removal of Bmal1 increases ROS levels in a variety of mammalian organs.26–30
Hence, it is possible to speculate that the reduction in cone viability observed in our previous study in mice lacking Bmal114 may have been due to a dysfunction in the circadian regulation of the cells’ antioxidant defenses. Unfortunately, in the mouse retina, cones comprise only 2% to 3% of the total photoreceptors; thus, performing in vivo mechanistic studies of these cells is challenging.
Recent studies have reported that 661W cells, which are a murine cone-like photoreceptor cell line developed by Tan et al.,31 are useful for investigating the fundamental aspects of cone biology in vitro.31,32 Using these cells, several studies have provided important insights into the molecular mechanisms regulating photoreceptor metabolism33–35 and death following exposure to oxidative stress.36,37
The aim of the present study was first to investigate whether 661W cells contain a functional circadian clock and then to explore whether the cells would be useful for investigating the role of the circadian clock in the modulation of their response to oxidative stress.
661W cells (RRID:CVCL_6240) were grown in Dulbecco’s Modified Eagle Medium (DMEM; Gibco, Life Technologies, Carlsbad, CA, USA) supplemented with 5% fetal bovine serum (Gibco) and 1% penicillin/streptomycin at 37°C in a 5% CO2 humidified atmosphere.37 Cells were seeded in 35 mm or 100 mm dishes, 96-well plates (Corning, Inc., Corning, NY, USA), or chamber slides (Lab-Tek, Inc., Grand Rapids, MI, USA) at a concentration of 1 × 104 to 5 × 105 cells in a volume of 0.2 to 5 mL of media and grown to approximately 50% to 90% confluence, depending on the experimental protocol.
Per2-luc, a plasmid that expresses the luciferase gene driven by the Per2 promoter, was used.38 Briefly, 1 μg of Per2-luc and 0.1 μg of pcDNA3 (which expresses the neomycin-resistant gene driven by the cytomegalovirus enhancer-promoter) was transfected by lipofectamine 2000 (Invitrogen, Waltham, MA, USA) into the 661W cells. The cultured 661W cells were then treated with a final concentration of 400 μg/mL geneticin and geneticin-resistant colonies were selected for bioluminescence verification. Cell lines that express luciferase activity in a circadian manner were identified by the bioluminescence emitted from 661W cells. Per2-luc-transfected 661W cells were cultured in DMEM containing 0.1 mM D-luciferase K salt (Molecular Imaging Products Co., Bend, OR, USA), and the bioluminescence emitted from the cells was monitored with either Lumicycle (Actimetrics, Wilmette, IL, USA; see details in Baba et al.39) or a charged-coupled device (CCD) camera (Stanford Photonics, Palo Alto, CA, USA; XR Mega 10Z; see details in Evans et al.40 and Baba et al.39). Briefly, the bioluminescence images of cultured 661W cells were obtained by a Zeiss AxioObserver Z1 microscope (Zeiss, Oberkochen, Germany) with an ×10 Fluar objective lens (Zeiss), Marzhauzer scanning stage with LUDL Mac 5000 controller, and a Stanford Photonics (Palo Alto, CA, USA) XR Mega 10Z cooled intensified CCD camera in a custom-built light-tight chamber maintained at 37°C. Bioluminescence rhythms recorded by Lumicycle were analyzed by Lumicycle analysis software (Actimetrics), and a peak of the Per2-luc rhythm was identified by Origin software (OriginLab Corp., Northampton, MA, USA; see details in Baba et al.41). Briefly, bioluminescence recordings obtained from Lumicycle were detrended by a 24-hour moving average subtraction method and smoothed by a 2-hour moving average. The circadian peak phase was determined as the highest point of the curve picked by Origin software. Per2-luc bioluminescence emitted from 661W cells was captured by a CCD camera every 30 minutes for three days. The intensity of the bioluminescence from consecutive captured images was analyzed with ImageJ software (National Institutes of Health [NIH], Bethesda, MD, USA).
A construct to knock out Bmal1 was purchased from Santa Cruz Biotechnology, Inc. (Dallas, TX, USA; BMAL1 CRISPR/Cas9 KO Plasmid, Santa Cruz sc-419206). The BMAL1 CRISPR/Cas9 KO plasmid is a pool of three different gRNA plasmids with the following sequences: sc-419206 A: Sense: 5′- TAGATAAACTCACCGTGCTA-3′; sc-419206 B: Sense: 5′-CTGCACGTACCCTGAGAATT-3′; sc-419206 C: Sense: 5′-TTACTAGGTACCTTCCATGA-3′. The Per2-luc 661W cells cultured on a 12-well plate were co-transfected with 0.5 μg of the BMAL1 CRISPR/Cas9 KO plasmid and 0.5 μg of the BMAL1-R HDR plasmid (Santa Cruz sc-419206-HDR). The plasmid included a puromycin resistance gene (used for the selection of colonies) and the red fluorescent protein (RFP) to detect the correct insertion of the plasmid in the genome. After selection in a medium containing puromycin (4 μg/mL), only cells with RFP signals were isolated. Then the removal of Bmal1 from the 661W cells was verified by western blotting using the BMAL1 antibody (Cell Signaling Technology, Danvers, MA, USA; cat. no. 14020; RRID:AB_2728705).
The plasmid pMSCV-Zeo was purchased from Addgene (Watertown, MA, USA; RRID:Addgene_75088). A 1.1-kb EcoRI to EcoRV fragment containing the expression cassette of the bleomycin-resistant gene driven by the PGK promoter was cut from the pMSCV-Zeo and cloned into the corresponding sites of pCMVSport6 to generate pSport6Zeocin. To re-express Bmal1 in the 661W-Bmal1-KO cells, the Bmal1 KO cell line was transfected by lipofectamine 2000 (Invitrogen, Waltham, MA, USA) with 250 ng of pCMVFlagBmal1 and 25 ng of pSport6Zeocin. Zeocin-resistant colonies were isolated and developed into cell lines. Bmal1 expression was verified by western blotting.
Cultured 661W cells were collected at different time points and the total RNA was isolated using TRIZOL (Life Technologies). cDNA was synthesized from isolated RNA using a High-Capacity RNA-to-cDNA Kit (Life Technologies). The quantitative polymerase chain reaction (Q-PCR) was performed with the CFX96 Touch Real-Time PCR Detection System (Bio-Rad Laboratories, Hercules, CA, USA) using iQ SYBR Green Supermix (Bio-Rad Laboratories). The primer sequences used to evaluate the gene expression were Per1 forward, 5′-TGAAGCAAGACCGGGAGA-3′ reverse, 5′-CACACACGCCGTCACATCAA -3′; Per2 forward, 5′-GAAAGCTGTCACCACCATAGAA-3′ reverse 5′-AACTCGCACTTCCTTTTCAGG-3′; Bmal1 forward, 5′-AACCTTCCCGCAGCTAACAG-3′ reverse 5′-AGTCCTCTTTGGGCCACCTT-3′; DBP forward 5′-CCTGAGGAACAGAAGGATGA-3′ reverse 5′-ATCTGGTTCTCCTTGAGTCTTCTTG-3′; Nrf2 forward, 5′-CGAGATATACGCAGGAGAGGTAAGA-3′ reverse 5′-GCTCGACAATGTTCTCCAGCTT-3′; 18S ribosomal RNA forward 5′-TTGTTGGTTTTCGGAACTGAGGC-3′ reverse 5′-GGCAAATGCTTTCGCTCTGGTC -3′.
Hydrogen peroxide (H2O2, Sigma-Aldrich, St. Louis, MO, USA) was diluted with double-distilled water to 1 M as a working solution, and 2 μl were added to 2 mL of the medium to make the final 1-mM concentration. Liproxstatin-1 (Lip1; Cayman Chemical, Ann Arbor, MI, USA) was dissolved in DMSO (47 mM) and further diluted with PBS to 200 μM. Ten microliters of this solution was added to 2 mL of the medium to make 2 μM of the final concentration.
The antioxidant capacity and glutathione peroxidase (GPx) activity were determined using commercially available assay kits (Antioxidant Assay Kit, Cayman Chemical cat. no. 709001; Glutathione Peroxidase Assay Kit, Cayman Chemical cat. no. 703102). The 661W and BKO cells were seeded and grown in 100-mm dishes with 5 mL of medium. When the cells reached 80% to 90% confluency, the medium was exchanged. Cells were washed with PBS and harvested using a cell scraper (CELLTREAT Scientific Products, Pepperell, MA, USA) at 26.5 and 38.5 hours after the medium exchange. Cells were then collected in a 1.5-mL tube, and the numbers of cells in each tube were counted using a cell counter (Bio-Rad TC20 Automated Cell Counter). Cells in the tubes were centrifuged (1000 × g for 10 min), and the cell pellet was homogenized in the extraction buffer included in the kits. After centrifuging, the supernatant was collected and the antioxidant capacity or GPx activity was measured according to the manufacturer’s protocol. The microplate reader (Cytation 3; BioTek Instruments, Inc., Winooski, VT, USA) was used to measure the absorbance at 750 and 405 nm for the antioxidant assay and at 340 nm for the GPx assay.
Cells were grown to near confluence in 35-mm dishes or 6-well plates (Falcon, Fisher Scientific, Waltham, MA, USA), washed with PBS, treated with trypsin, and transferred to 15-mL conical centrifuge tubes (Falcon) with the addition of 5 mL of medium. After centrifugation (400 x g for 5 min), the cell pellets were resuspended in 5 mL of PBS. Cell pellets obtained after another centrifugation (400 x g for 5 min) were lysed with RIPA buffer (Boston BioProducts, Inc., Milford, MA, USA) and supplemented with a protease inhibitor cocktail (cat. no. 5871S, Cell Signaling Technology). Cell lysates were cleared by centrifugation (17,000 x g for 30 min), and aliquots were mixed with Laemmli buffer, 6X SDS-Sample buffer, (cat. no. BP-11R, Boston Bioproducts, Inc.), heated to 950°C, loaded, and run on a Criterion Midi Protein Gel with the Criterion Vertical Electrophoresis Cell System (Bio-Rad Laboratories). Proteins were transferred from gels onto an Amersham HyBond PVDF membrane (Cytiva Life Sciences, Marlborough, MA, USA) using the Bio-Rad Trans-Blot Turbo Transfer system. Membranes were washed with 1X Tris buffered saline (TBS), diluted from a 20X TBS stock solution (Boston Bioproducts, Inc.), for 5 minutes followed by blocking in 5% non-fat dry milk or bovine serum albumin (BSA) (Boston Bioproducts, Inc.) in TBS. The primary antibody in TBST (0.1% Tween-20 in 1X TBS) was then added to the membrane and placed on a rocking platform at 4°C overnight. The membrane was washed in TBST three times for 10 minutes each time on a rotating platform at room temperature before the secondary antibody in TBST was added. Fluorescence-conjugated antibodies were purchased from Fisher Scientific; they included Invitrogen’s goat-anti-rabbit Alexa Fluor Plus 800 (Thermo Fisher Scientific, Inc.; RRID:AB_2633284) or goat-anti-rabbit Alexa Fluor Plus 680 (Thermo Fisher Scientific, Inc.; RRID:AB_2633283), depending on the experiment. After an 1-hour incubation at room temperature, the membrane was washed in TBST three times for 10 minutes each time, after which imaging was done with the LI-COR Odyssey Fc Imaging system (LI-COR, Lincoln, NB, USA).
661W cells were seeded and cultured in chamber slides (Lab-Tek, Inc.) for immunohistochemical studies. Cells were fixed in paraformaldehyde (PAF) 4% and washed with PBS. Cells were incubated for 45 min in 1% BSA and 0.4% Triton-X100 in PBS to permeabilize the membranes and block unspecific binding. Cells were then incubated overnight at 4°C with primary BMAL1 antibodies (1:1500; cat. no. 14020, Cell Signaling Technology), washed in PBS, and incubated for 2 hours at room temperature in secondary antibodies (anti-rabbit conjugated with Alexa Fluor 488, 1:1000). After washing in PBS, slides were cover-slipped with Vectashield (Vector Laboratories, Newark, CA, USA) and cells were visualized with a fluorescence microscope (Zeiss LSM700).
Data were analyzed with a one-way or two-way ANOVA and the Tukey multiple comparison test using SPSS software (IBM; RRID:SCR_016479). A simple paired t-test was performed with the Microsoft Excel (RRID:SCR_016137) data analysis program. The rhythmicity of the oxidative stress response was analyzed by the Nitecap program (https://nitecap.org/: developed by the University of Pennsylvania Perelman School of Medicine).42
Cultured 661W cells were grown in a culture dish and collected when they were confluent. RNA was isolated from these cells; Per1, Per2, Bmal1, and 18S mRNAs were amplified; and analyzed by electrophoresis. The expression of these genes in the mouse brain, retina, and retinal pigment epithelium (RPE) was also analyzed by electrophoresis to serve as reference points. The results showed that clock genes were expressed in the 661W cells, retina, RPE, and brain (Figure 1A). To examine the circadian oscillation in the 661W cells, the cells were collected 20.5, 26.5, 32.5, and 38.5 hours after the cells were resynchronized by a medium exchange, and the expression levels of Per2, Bmal1, and DBP mRNAs were measured. The Per2 and DBP mRNAs were expressed in a circadian manner (Figure 1B and C, one-way ANOVA, p < 0.05), with peak expression at 20.5 hours. Bmal1 also showed circadian expression (Figure 1D, one-way ANOVA, p < 0.05) with peak expression at 32.5 hours. The bioluminescence emitted from the 661W cells transfected with the Per2-luc reporter also showed a circadian rhythm (Figure 1E). The circadian peak of Per2-luc bioluminescence in the 661W cells occurred 20.47 ± 0.21 hours after the medium exchange, and the average circadian period was 23.18 ± 0.15 hours (mean ± SEM, n = 100). The bioluminescence intensity captured by the CCD camera for three days also showed a circadian rhythm (Figure 1F, peak phase 20.23 hours, period 22.02 hours). A single cell was selected in the area of the CCD image by ImageJ software (RRID:SCR_003070), and a robust Per2-luc circadian rhythm was also observed for a single 661W cell (Figure 1G and H: cell 1 peak: 24.36 hours, period: 20.08 hours; cell 2 peak: 18.43 hours, period 23.90 hours).
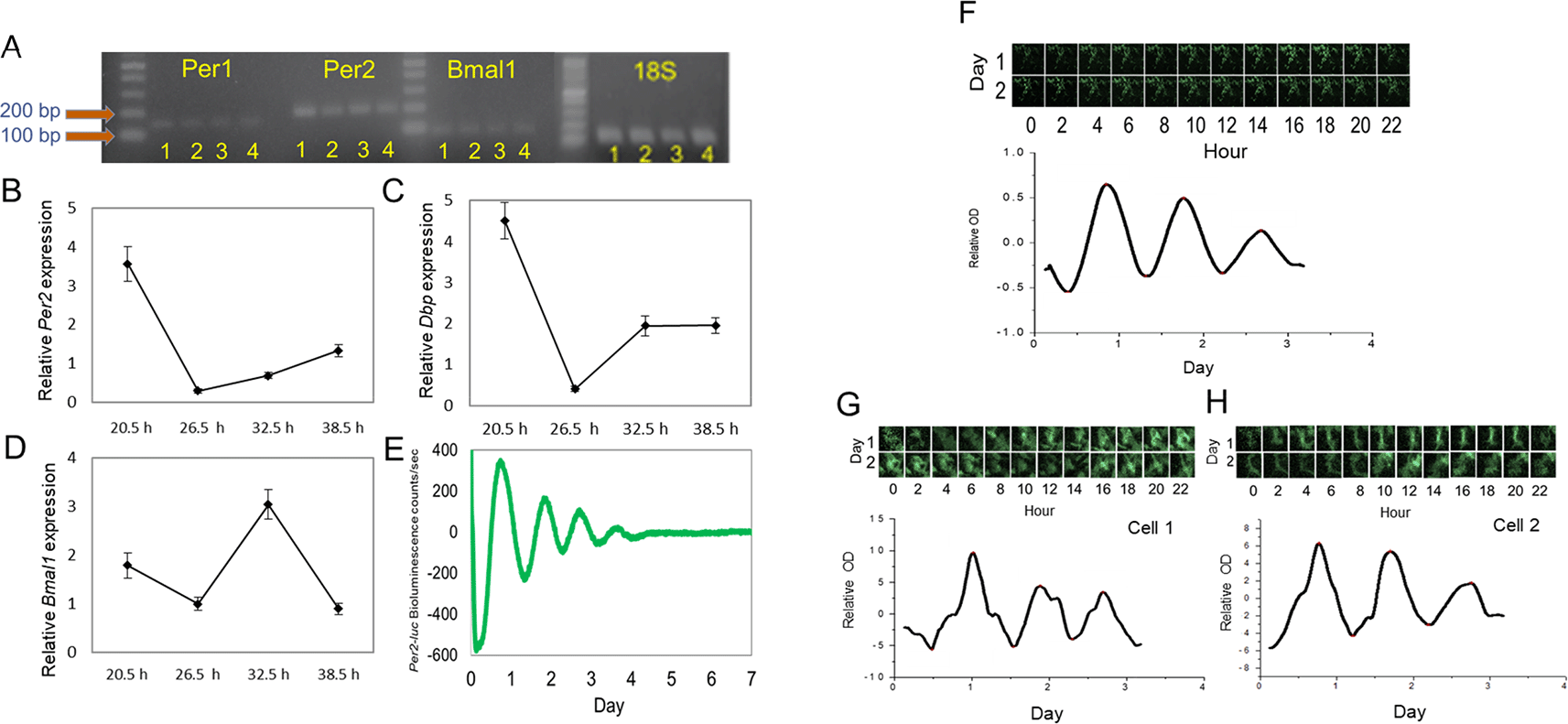
(A) Per1, Per2, and Bmal1 mRNA was amplified in mouse brain (1), retina (2), RPE (3), and 661W cells (4). (B to D) Circadian expression of Per2, Bmal1, and DBP mRNA in 661W cells at four different time points (one-way ANOVA, p < 0.05 in all cases, n = 6). (E) Representative Per2-luc bioluminescence circadian rhythm in 661W cells (detrended data) measured by Lumicycle. (F) Per2-luc bioluminescence from 661W cells measured with a CCD camera for 2 days; the bioluminescence rhythm was analyzed. (G to H) A single cell’s Per2-luc bioluminescence was measured with a CCD camera.
Several Bmal1 KO (BKO) 661W cell lines were generated using the CRISPR-cas9 gene-editing tool. Western blot analysis showed the successful removal of Bmal1 in cell lines 2, 3, 4, 8 and 9 (Figure 2A). A long-exposure image also confirmed the loss of BMAL1 expression. We selected the cell line with the least signal to be used in our experiments. The deletion of Bmal1 in this line was verified by immunohistochemical studies (Figure 2B). Consistent with these results, we did not observe any rhythmicity in the expression levels of Per2, Bmal1, or DBP mRNA in the BKO cells (one-way ANOVA, p > 0.1 in all cases; Figure 2C to E) or in the Per2-luc bioluminescence (Figure 2F).
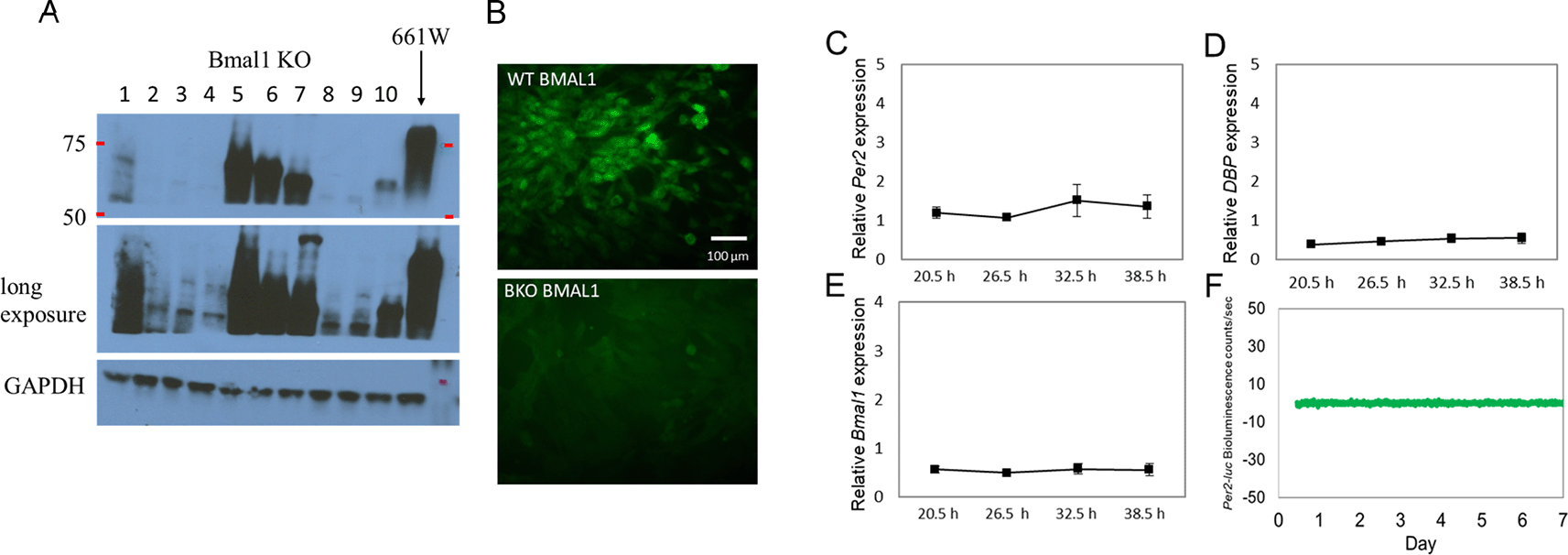
(A) Western blot indicating the successful knockout of BMAL1 in multiple cell lines (i.e., lines 2, 3, 4, 8, and 9); others show a truncated Bmal1 (i.e., lines 5, 6, 7, and 10). (B) BMAL1 immunoreactivity was detected in 661W cells (upper panel), but not in a BKO cell line (line 4) (lower panel). (C to D) Loss of rhythmicity in Per2, DBP, and Bmal1 mRNA levels in BKO cells (one-way ANOVA, p > 0.05 in all cases, n = 6). (F) 661W-BKO cells no longer exhibit Per2-luc bioluminescence rhythm (detrended data) as observed in Figure 1E.
The 661W and BKO cells were cultured and then subjected to an oxidative stress challenge by adding 1 mM of H2O2 to the cultured cells at six-hour intervals starting from the first peak phase of the Per2-luc (Figure 3A) for two consecutive circadian cycles (from 20.5 to 62.5 hours after medium exchange). For the control groups were treated with the same volume of double distilled water (n = 6 to 8 for each time point) and the survival rate was normalized with the control value. The 661W cells showed a clear circadian rhythm in their responses to the oxidative stress challenge (Figure 3B, one-way ANOVA, p < 0.05, Jonckheere-Terpstra-Kendall (JTK) algorithm p < 0.001: period 24.0 hours: amplitude 0.233, n = 7 to 8). The cells were most resistant to the oxidative stress challenge at 26.5 and 50.5 hours (second cycle) and least resistant at 38.5 and 62.5 hours (second cycle). In the BKO cells, the survival rate was greatly reduced (only ~30% survived the H2O2 treatment, two-way ANOVA, p < 0.01 between groups) and no circadian variation was observed (one-way ANOVA, p > 0.05; JTK algorithm p > 0.05, Figure 3B).
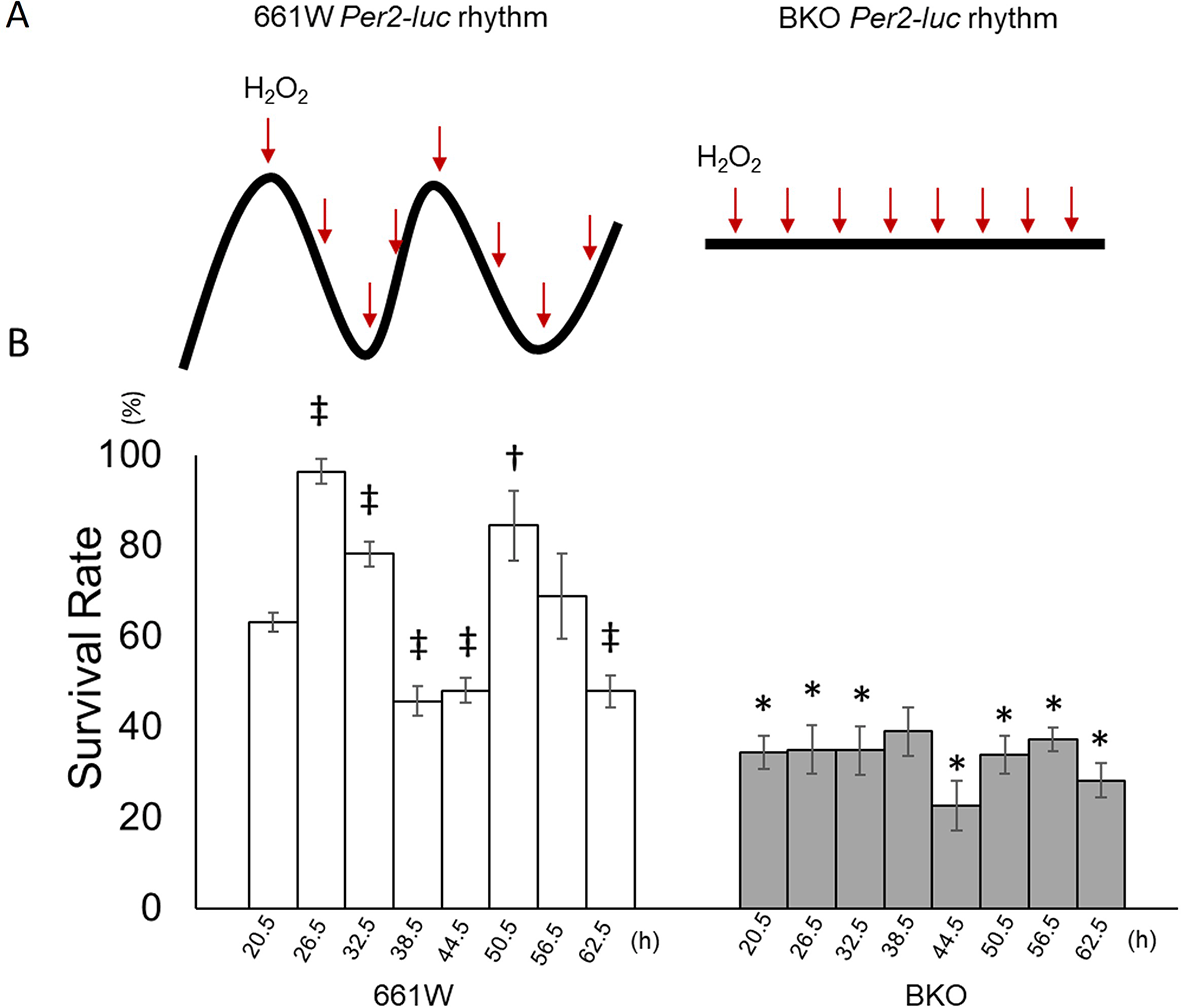
(A) Schematic illustration of experimental procedures. Cells were treated with H2O2 at 6-h intervals from the peak phase of the Per2-luc rhythm for two circadian cycles. BKO cells were treated with H2O2 at the same time points as in 661W cells. (B) The survival rate following exposure to H2O2 (1 mM) at different circadian times (from 20.5 to 60.5 h) showed a clear circadian rhythm. The survival rate was highest at 26.5 h and lowest at 38.5 h during the first cycle after medium exchange and at 50.6 h and 60.5 h, respectively, during the second cycle. BKO cells did not show circadian variation in oxidative stress sensitivity and had a lower survival rate compared with 661W cells. (Mean ± SEM, n = 7 to 9, two-way ANOVA followed by Tukey multiple comparison test, †p < 0.05, ‡p < 0.05 compared with 661W cells at 20.5 h, *p < 0.05 compared with same time point for 661W cells).
The nuclear factor erythroid 2–related factor 2 (Nrf2) is a well-known regulator for antioxidant response elements,43 and the expression of Nrf2 is regulated by the circadian clock via E-box elements.44–46 Hence, we hypothesized that the circadian variation in the sensitivity to oxidative stress was mediated by the circadian regulation of Nrf2. Indeed, Nrf2 mRNA showed a circadian expression in 661W cells (Figure 4A, one-way ANOVA, p < 0.05), whereas in the BKO cells, the expression level of Nrf2 mRNA was arrhythmic and significantly decreased. We measured the antioxidant capacity in 661W and 661W-BKO cells at the time of maximum and minimum cell survival to the oxidative stress challenge (i.e., 26.5 and 38.5 hours). In the 661W cells, the antioxidant capacity at 26.5 hours was fourfold higher than the antioxidant capacity at 38.5 hours (two-way ANOVA followed by the Tukey multiple comparison test, p < 0.05; Figure 4B). The antioxidant capacity of the 661W-BKO cells was significantly reduced (~50%) with respect to what we observed in the 661W cells at 26.5 hours (p < 0.05). We also observed a slight difference in the antioxidant capacity in the BKO cells between 26.5 and 38.5 hours (p < 0.05; Figure 4B). Of the many antioxidants present in the cells, GPx was a possible candidate for the mediation of the circadian response to oxidative stress because GPx is an intracellular antioxidant that enzymatically reduces H2O2 to H2O.47 Thus, we decided to investigate whether GPx activity was also regulated by the circadian clock in the 661W cells. As shown in Figure 4C, the GPx activity was higher at 26.5 hours and lower at 38.5 hours in the 661W cells (two-way ANOVA followed by Tukey multiple comparison test, p < 0.05; Figure 4C). In the BKO cells, the GPx activity was lower at 26.5 hours compared with in the 661W cells (two-way ANOVA followed by Tukey multiple comparison test, p < 0.05), and no difference was observed between the level of GPx activity at 26.5 and 38.5 hours.
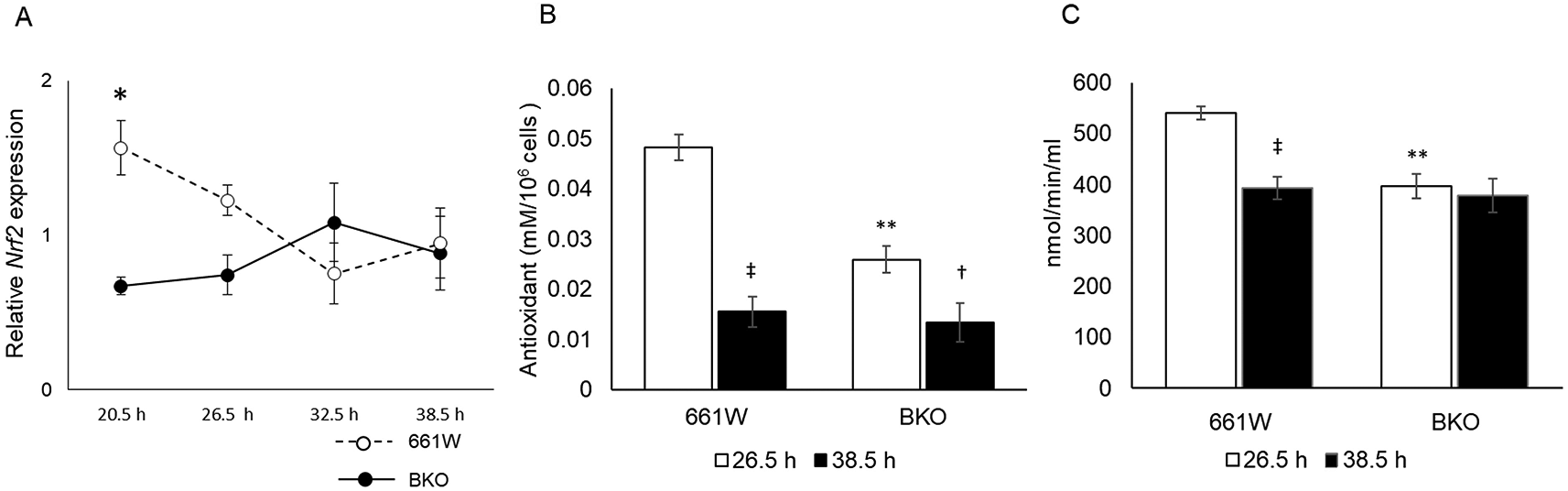
(A) Circadian expression of Nrf2 was rhythmic in 661W cells but not in BKO cells (two-way ANOVA, p < 0.05 between groups on the Tukey multiple comparison test, *p < 0.05, one-way ANOVA 661W cells: p < 0.05; BKO cells: p > 0.05, n = 6). (B) Circadian variation in antioxidant capacity was observed in both WT and BKO cells, although antioxidant capacity of BKO cells was lower than of 661W cells at 26.5 h (two-way ANOVA followed by Tukey multiple comparison test, n = 6, **p < 0.01 661W cells vs. BKO cells, †p < 0.05, ‡p < 0.01 26.5 h vs. 38.5 h). (C) Circadian fluctuation of GPx activity was observed in WT 661W cells but not in BKO cells (two-way ANOVA followed by Tukey multiple comparison test, n = 5 to 6, **p < 0.01 WT cells vs. BKO cells, ‡p < 0.01 26.5 h vs. 38.5 h).
Ferroptosis is a cell death process that is driven by the accumulation of iron-dependent lipid peroxidation.48 GPx plays an important role in this cell death pathway.49 We hypothesized that ferroptosis mediated the cell death process in 661W and BKO cells after H2O2 exposure. To explore this theory, we investigated whether the ferroptosis inhibitor liproxstatin-1 (Lip1) could prevent cell death from oxidative stress. The 661W and BKO cells were cultured, and pretreated with Lip1 (2 μM) 30 minutes prior to the H2O2 treatment at 26.5 and 38.5 hours. As shown in Figure 5, treatment with Lip1 increased the survival of BKO cells at 26.5 hours (Figure 5A) and almost completely prevented cell death at 38.5 hours (Figure 5B) in both genotypes (two-way ANOVA followed by Tukey multiple comparison test, p < 0.01).
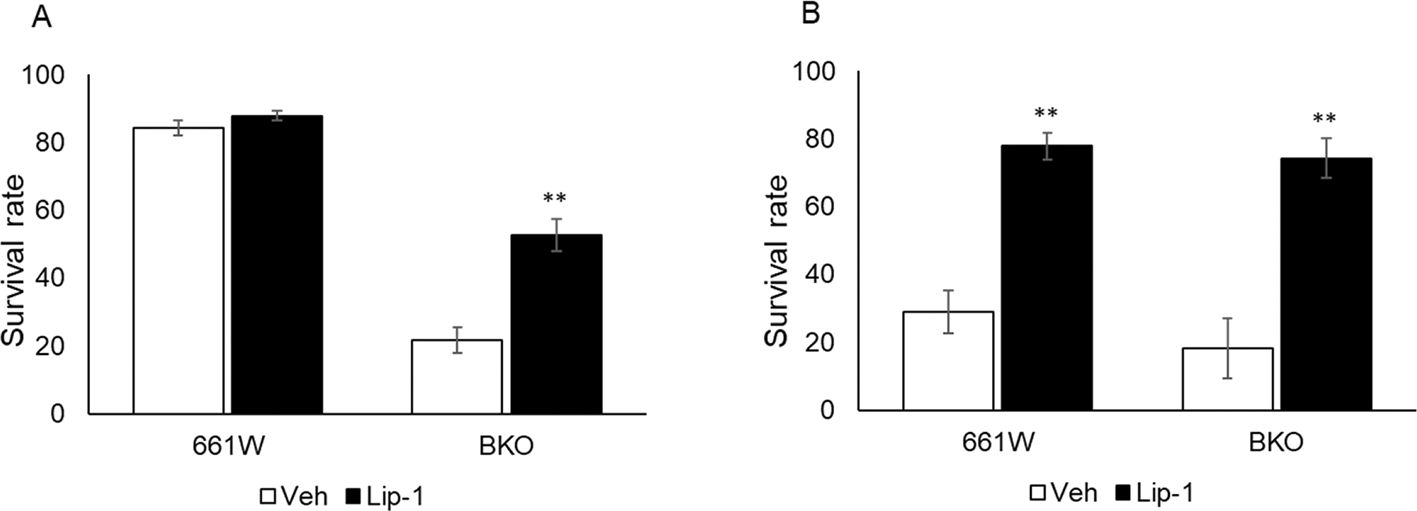
(A) Treatment of Lip1 increased cell viability by approximately 30% in BKO cells at 26.5 h after medium exchange. (B) Lip1 treatment significantly increased cell viability in both cell types from H2O2-induced cell death at 38.5 h after medium exchange (two-way ANOVA followed by Tukey multiple comparison test, n = 6, **p < 0.01 control vs. Lip1).
To confirm that the effects we observed in the BKO cells were due to the removal of Bmal1, we rescued Bmal1 in the BKO cells by transfecting the Bmal1 construct into the BKO cells. The rescued cell lines were confirmed by western blotting (Figure 6A). Stable BKO-Bmal1–rescued cells were cultured and exposed to an oxidative stress challenge as previously described (Figure 3). Our data indicate that the rescued Bmal1 in the BKO cells significantly increased the cell survival rate (10% – 20%) during the oxidative stress challenge at 20.5, 26.5, and 32.5 hours (two-way ANOVA followed by Tukey multiple comparison test, *p < 0.05; Figure 6B).
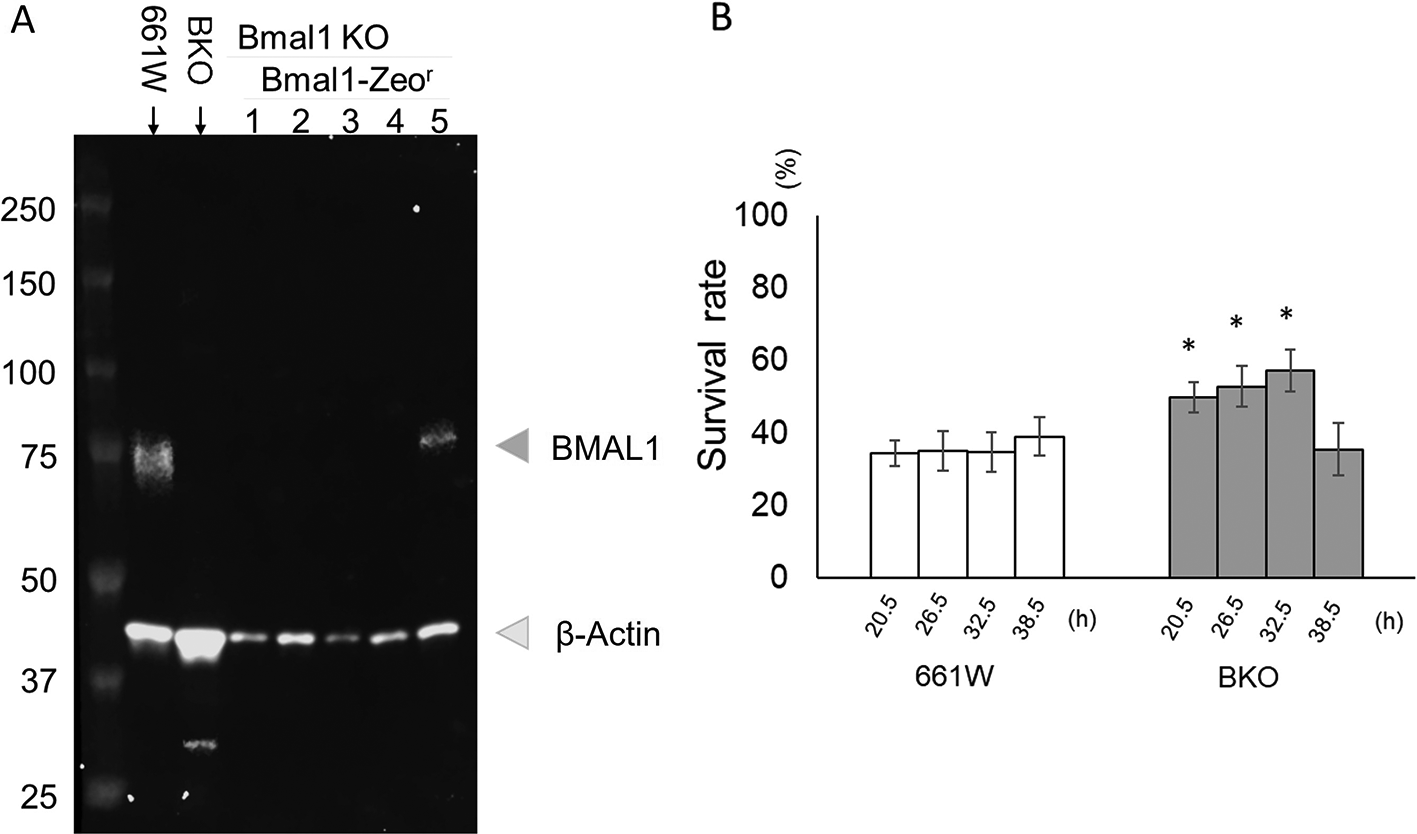
(A) Western blot indicating the successful re-expression of BMAL1 in the BKO cells (line #5). (B) Bmal1 rescue restored circadian variation in the sensitivity to oxidative stress in BKO cell (two-way ANOVA followed by Tukey multiple comparison test, n = 6 to 12, *p < 0.05 BKO rescued cell vs. BKO cell).
The effects of circadian dysfunction caused by genetic and environmental manipulations of the retina have garnered considerable interest in recent years. As previously mentioned, experimental evidence indicated that the cone photoreceptors are very susceptible to circadian disruption.4,5,12 However, the lack of a suitable cell model has hampered progress in understanding the molecular mechanism(s) with which the circadian clock may regulate biological functions in these cells. In the present study, we have demonstrated that the cone-like photoreceptor cell line 661W, contains a functional circadian clock; that this circadian clock modulated the response of these cells to an oxidative stress challenge; that the mechanisms by which the circadian clock modulated this response involved the regulation of GPx activity; and that the cell death we observed after H2O2 exposure was due to ferroptosis. We also observed that removal of the clock gene Bmal1 from the cell abolished the circadian response and that the rescue of Bmal1 in the BKO cells increased cell survival after oxidative stress. Thus, we believe that the 661W cell line is a useful new tool for investigating the action of the circadian clock in regulating photoreceptor (cone) biology.
ROS are by-products of metabolic processes and are produced according to the metabolic needs of cells.50 In the retina, the high level of ROS production by photoreceptors occurs during the day when the photoreceptors are actively involved in the phototransduction of light signals.51,52 Previous studies suggested that circadian dysfunction increases intracellular ROS level26–30 and that the accumulation of ROS and dysfunction of ROS homeostasis in retinal cells leads to many types of retinal diseases.20 Antioxidants are endogenous products that scavenge excessive ROS. This ROS scavenging system is crucial for cell maintenance and survival.22 Previous studies have shown that the expression patterns of antioxidant enzymes (i.e., GPx, catalase, peroxiredoxin, and superoxide dismutase) are controlled by the circadian clock.53–57 Therefore, a functional circadian clock may provide an important protective function by synchronizing the production of the antioxidant response with the time of maximum ROS production, thus reducing the numbers of ROS in a cell. Our new data supported this notion by demonstrating that in 661W cells, the circadian clock can modulate the response to oxidative stress by regulating the antioxidant capacity (Figures 3 and 4B).
A number of studies have suggested that the involvement of the circadian clock in the regulation of antioxidant elements is mediated through Nrf2.57,58 Indeed, previous investigations have shown that the circadian expression of Nrf2 is under the direct control of the circadian clock via the E-box present on the promoter region of this gene and that Nrf2 shows a similar pattern of expression of Per1 and Per2.12,44 Our results agree with these previous studies, showing that Nrf2 mRNA is rhythmically transcribed with a similar rhythmic pattern of Per2 in 661W cells but not in BKO cells.
One of the most surprising results obtained in our study regarding the antioxidant capacity of 661-BKO cells in the presence of a circadian rhythm (although at a much lower amplitude than 661W cells) (Figure 4A). A previous study showed that peroxiredoxins, a group of antioxidant proteins, may have a circadian rhythm in redox cycles in red blood cells,56 which have no nucleus and therefore cannot generate a circadian oscillation via the transcriptional translational feedback loops’ clock mechanism.59 Additionally, another recent study has reported that circadian oscillation in the transcriptome and proteome of mammals can be present in the fibroblast and in the mouse liver even when Bmal1 has been removed.60 Although the results reported in these studies are controversial,61,62 our new data may suggest that in some situations, the removal of Bmal1 may not completely abolish circadian oscillations (Figure 4B). However, it is important to notice that although the antioxidant capability has a circadian variation in 661W-BKO cells, the changes did not extend to changes in the GPx antioxidant capability (Figure 4C) and, more importantly, did not translate to cell survivability after an oxidative stress challenge (Figure 3B).
Ferroptosis is a newly discovered nonapoptotic cell death cascade that is characteristic of the iron-dependent oxidation of phospholipids.47 Ferroptosis is activated by the dysregulation of the GPx antioxidant defense mechanism, which causes lipid peroxidation and cell death.63 Circadian expression of GPx has been reported in many organs, including the mouse retina,44,57 and accumulating evidence suggests that the dysregulation of GPx causes retinal diseases and blindness.64,65 Our results showed that Lip-1 (inhibiting lipid peroxidation to prevent ferroptosis activation66) prevents cell death from oxidative stress at 38.5 hours in both genotypes, thus suggesting that the cell death that follows H2O2 exposure is probably due to ferroptosis. However, it may be that other cell death pathways (i.e., apoptosis, necroptosis67,68) are involved, since Lip-1 only partially prevented cell death in BKO cells at 26.5 hours.
Finally, it is important to mention that in vivo studies have shown that the magnitude of oxidative stress damage (light-induced photoreceptor damage) is greatly affected by the time of the day. Nocturnal rodents are three to four times more susceptible to light damage at night than during the day.69–71 The circadian dependence of light-induced photoreceptor damage appears to involve changes in the cAMP levels.72 Our new data expands these previous results by suggesting that a change in GPx may be involved in the modulation of the circadian dependence of light-induced photoreceptor damage.
Our work supports the notion that the presence of a functional circadian clock and its ability to modulate the response to an oxidative stress is the undelaying mechanism that may protect cones during aging.
KB, T-CS, VG, AS, AD and JD performed experiments and analyzed experimental results. KB and GT planned/designed the studies, provided data/figures, and wrote the manuscript.
Figshare: The circadian clock mediates the response to oxidative stress in a cone photoreceptor–like (661W) cell line via regulation of glutathione peroxidase activity, https://doi.org/10.6084/m9.figshare.20537982.v1. 73
• qPCR CT values for circadian gene expressions in 661W cells
• Figure 1 clock gene expressions in 661W cells
Figshare: The circadian clock mediates the response to oxidative stress in a cone photoreceptor–like (661W) cell line via regulation of glutathione peroxidase activity, https://doi.org/10.6084/m9.figshare.20538408.v1. 74
• The peak phases and circadian periods of Per2-luc biouminescence rhythms in 661W cells
• Per2-luc phases and periods in 661W cells
Figshare: The circadian clock mediates the response to oxidative stress in a cone photoreceptor–like (661W) cell line via regulation of glutathione peroxidase activity, https://doi.org/10.6084/m9.figshare.20538621.v1. 75
• Slide 1: CCD recording from population of 661W cells. Slide 2-4: Single cell recording. Slide 4 may show circadian rhythms of multiple cells since the first peak shows two peaks
• Per2-luc bioluminescence from 661W cells measured with a CCD camera
Figshare: The circadian clock mediates the response to oxidative stress in a cone photoreceptor–like (661W) cell line via regulation of glutathione peroxidase activity, https://doi.org/10.6084/m9.figshare.20538711.v1. 76
• qPCR CT values for circadian gene expressions in Bmal1 KO cells
• Figure 2 Circadian gene expressions in BKO cells
Figshare: The circadian clock mediates the response to oxidative stress in a cone photoreceptor–like (661W) cell line via regulation of glutathione peroxidase activity, https://doi.org/10.6084/m9.figshare.20538885.v1. 77
• The cell survival rate following exposure to H2O2 or vehicle control treatment in 661W and BKO cells
• Figure 3 Cell viability from oxidative stress challenge
Figshare: The circadian clock mediates the response to oxidative stress in a cone photoreceptor–like (661W) cell line via regulation of glutathione peroxidase activity, https://doi.org/10.6084/m9.figshare.20538975.v1. 78
Figshare: The circadian clock mediates the response to oxidative stress in a cone photoreceptor–like (661W) cell line via regulation of glutathione peroxidase activity, https://doi.org/10.6084/m9.figshare.20539026.v1. 79
• Data sets for Nrf2 circadian expression in 661W and BKO cells and circadia variation for GPx activity level
• Figure 4 A and C data set Nrf2 expression and GPx activity level in 661W and BKO cells
Figshare: The circadian clock mediates the response to oxidative stress in a cone photoreceptor–like (661W) cell line via regulation of glutathione peroxidase activity, https://doi.org/10.6084/m9.figshare.20539398.v1. 80
• The spectrometry counts obtained from antioxidant assay in 661W and BKO cells
• Figure 4 B data set Measurement of antioxidant capacity in 661W and BKO cells
Figshare: The circadian clock mediates the response to oxidative stress in a cone photoreceptor–like (661W) cell line via regulation of glutathione peroxidase activity, https://doi.org/10.6084/m9.figshare.20539485.v1. 81
• The survival rate after Lip1 or vehicle treatment in 661W and BKO cells at two time points
• Figure 5 The survival rate after pretreatment of Lip1 followed by oxidative stress challenge
Figshare: The circadian clock mediates the response to oxidative stress in a cone photoreceptor–like (661W) cell line via regulation of glutathione peroxidase activity, https://doi.org/10.6084/m9.figshare.20539737.v1. 82
• The data set for the survival rate in Bmal1 rescued cells following oxidative stress challenge
• Figure 6 The cell viability in Bmal1 rescued cells following oxidative stress challenge
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The authors would like to thank Dr. Muayyad Al-Ubaidi (Department of Biomedical Engineering, University of Houston) for kindly donating the 661W cells.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Retina, circadian rhythms, opsins, cone photoreceptor.
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: retina, cornea, opsins, circadian rhythms
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 21 Nov 22 |
read | read |
|
Version 1 20 Sep 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)