Keywords
spontaneous remission, acute lymphoblastic leukemia, lymphoblastic lymphoma, relapse
This article is included in the Oncology gateway.
This article is included in the Cell & Molecular Biology gateway.
spontaneous remission, acute lymphoblastic leukemia, lymphoblastic lymphoma, relapse
The partial or complete, temporary or definitive disappearance of a malignant pathology without any specific treatment is referred to as spontaneous remission (SR) or regression of a cancer. For solid tumors, the expression “spontaneous regression” is used, whereas for leukemias, we speak of “spontaneous remission”.1,2 SR has been reported in various hematological malignancies, for example: in adult T-cell lymphoma,3 in chronic lymphocytic leukemia (CLL),4 in myelodysplastic syndrome (MDS),5 in acute myeloid leukemia (AML), rarely in acute lymphoblastic leukemia (ALL) in children and exceptionally in adults.2,6,7 SRs are often related to infections or other immune stimulators, resulting in potent immune activation playing a crucial role in controlling the leukemic clone.8,9 Nevertheless, the underlying mechanism of this phenomenon is not clearly described.
In this article, we report the case of a patient consulting for appendicular syndrome who was found to have blastic invasion during the preoperative workup, which regressed spontaneously during his hospitalization.
We report the case of a 40 year old man from Martinique with no past medical or surgical history who presented to the Emergency Department complaining of abdominal pain and fever. On abdominal ultrasound, an appendicular abscess was noted and on the count blood cells (CBC) the diagnosis of acute leukemia was made with leucopenia (white blood cells (WBCs) = 1.6×109/L) and 12% of circulating blast cells. His bone marrow aspiration was hypercellular with infiltration by 65% of undifferentiated blast cells (Figure 1). The patient did not undergo surgery due to the discovery of leukemia and the risks associated with the surgery in the context of anticipated aplasia, so the patient was put on first-line antibiotic therapy: cefepime (2 g × 2/day) and ciprofloxacin (750 mg × 2/day) for 7 days and transferred to the Gustave Roussy Institute (GRI) on December 14 for the management of his leukemia.
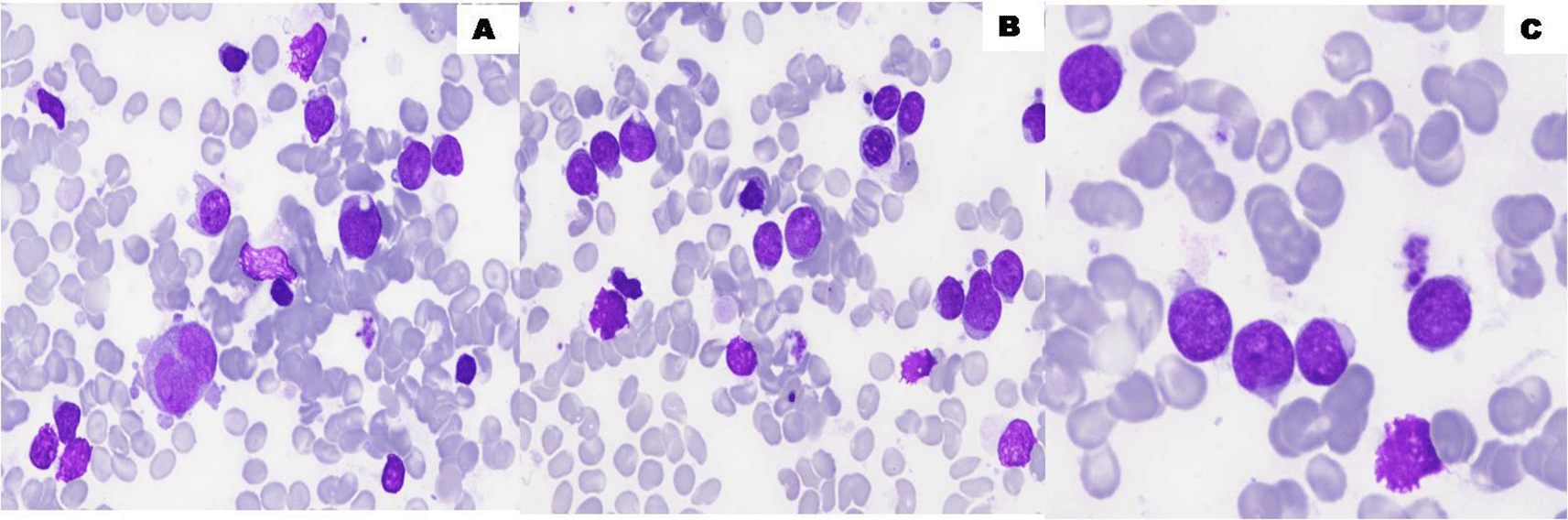
The bone marrow aspirate smears show hypercellular bone marrow (A, B) with infiltration by 65% of undifferentiated blast cells (C) (100×, May-Grünwald Giemsa stain). ALL (acute lymphoblastic leukemia).
On the hematological level, the evolution at the GRI was marked by a regression of the rate of circulating blasts and improvement of the leucopenia.
The CBC showed anemia (Hb 10.5g/dL), leucopenia (WBC 1.9*109/L) and normal rate of platelet (425*109/L). The differential leukocytic count showed neutropenia (0.8×109/L) and 2% of blast cells detected in a blood film. Repeat bone marrow aspiration revealed a marrow with moderate richness characterized by a hyperplasia of the erythroblastic compartment (55% among medullary figurative elements) associated with moderate signs of dyserythropoiesis and an excess of undifferentiated blasts (12%). The blast cells were small with agranular rim of cytoplasm and very high nucleocytoplasmic ratio. The nucleus has fine chromatin with one to two nucleoli (Figure 2).
The bone marrow aspirate smears show hyperplasia of the erythroblastic compartment with 55% erythroblast (A, B). Undifferentiated blast cells comprise 12% of bone marrow cellularity (C) with moderate signs of dyserythropoiesis (100x, May-Grünwald Giemsa stain).
Flow cytometry of the bone marrow aspirate revealed blasts positive for CD45 dim (15%), immaturity marker: CD34+ (85%), CD117+ (22%), HLADR+ (40%) and myeloid marker: CD33+ (75%), cCD13+ (68%), CD11b (61%) and expressing lymphoid T marker: CD5+ (67%), CD7+ (85%), cCD3+ (74%) (Figure 3), suggesting the possibility of an early T-cell acute lymphoblastic leukemia (ETP-ALL) according to the 2016 WHO acute leukemia classification.10 We also note a hyperplastic erythroblastic contingent: CD36+ (47%), Glycophorin A+ (26%), in agreement with the cytology. Molecular cytogenetic technique, such as Fluorescent in situ hybridization (FISH) showed a trisomy 8. Three days later a bone marrow aspiration was released and ETP-ALL was confirmed.

ALL (acute lymphoblastic leukemia); CD (cluster of differentiation); PE (phycoerythrin).
At GRI, the diagnosis of appendicitis without signs of complication was confirmed. After one week of observation, abdominal pains persisted and the appendicular syndrome did not regress in spite of an antibiotic therapy, requiring surgical intervention on December 20. The patient underwent a laparoscopic appendectomy, without postoperative anomalies.
At the same time, we noted a progressive normalization of the hemogram, only the anemia remained. Indeed, on December 31, CBC showed Hb 10.6 g/dL (anemia) and normal rate of WBC 4.2×109/L and platelet (432×109/L). The myelogram showed a rich, polymorphic, slightly erythroblastic marrow. The blastic infiltration seemed less important, evaluated at 4%. Therefore, no cytological aspect of acute leukemia was noted on this sample.
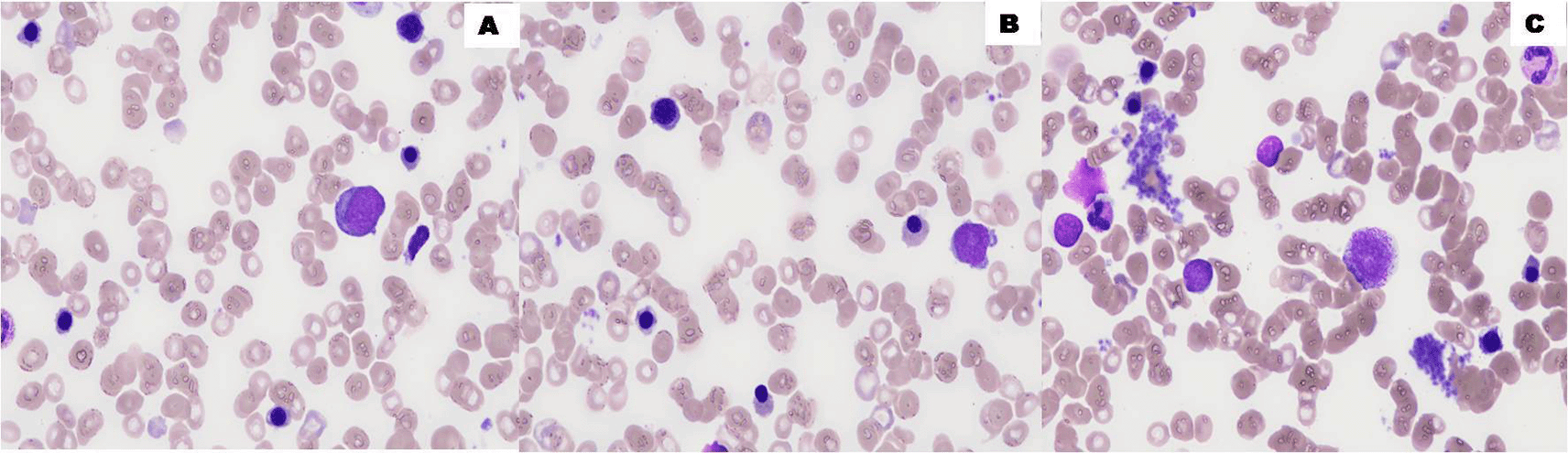
Two weeks later, CBC showed Hb 11.3g/dL, WBC 6.9×109/L and the differential leukocytic count showed 4.4×109/L neutrophils, 1.4×09/L lymphocytes, 0.9×109/L monocytes and 0.2×109/L eosinophils with no blast cells detected in a blood film. A bone marrow aspiration revealed a stable blastosis compared to the previous myelogram with 5% blasts and cytogenetic analysis also confirmed a cytogenetic remission (Figure 4).

Bone marrow aspirate smears show maturing hematopoiesis with no increased blasts (A,B) (100×, May-Grünwald Giemsa stain).
In view of the patient's clinical improvement and the SR of his acute leukemia, the patient was discharged from the hospital. He underwent a human leukocyte antigen (HLA) typing in order to search for a donor on file in case of the need for a marrow transplant in the event of relapse.
Nine months from first documented SR of the ALL, the patient relapsed. His bone marrow aspiration revealed a poor bone marrow with hyperplasia of the erythroblastic compartment (43% among medullary figurative elements) associated with moderate signs of dyserythropoiesis and an excess of undifferentiated blasts (50%) (Figure 5). Flow cytometry of the bone marrow aspirate revealed an early T-cell acute lymphoblastic leukemia (Figure 6). So, he received intensive chemotherapy according to the following protocol: daunorubicin (60 mg/m2 at day (D)1, D2, D3, D8 and D9) + cytarabine (30 mg/m2/D at D2 and D6) + aracytin (1,000 mg/m2/D at D1 and D6). Five months later, he underwent a placental blood allograft after conditioning with cyclophosphamide (50 mg/kg at D6), fludarabine (200 mg/m2/D at D6 and D2), total body irradiation (2 Gy at D1) but relapsed in the following year. Administration of two courses of cytarabine (1 g/m2 at D1 to D5) + clofarabine (30 mg/m2 at D2 to D6) was initiated but the patient remained very cytopenic without blast cells for another month to relapse again and died after two weeks.

The bone marrow aspirate smears show a poor bone marrow (A,B) with hyperplasia of the erythroblastic compartment (43%) and an excess of undifferentiated blasts (50%) (C) (100×, May-Grünwald Giemsa stain).
Acute leukemia with spontaneous remission has been reported since 1878.1 A PubMed (RRID:SCR_004846) search using the terms “acute leukemia”, “remission”, “spontaneous”, including only articles written in English excluding children, yielded a total of 49 articles that were reviewed and 60 cases reported of acute leukemia with SR. A total of 76% of these patients had an associated infection and 45% of them received a blood product transfusion. Less common associations were growth colony stimulating factor (G-CSF), steroids, hydroxyurea, termination of pregnancy, gonadotropin releasing hormone (GnRH), tumor lysis syndrome, discontinuation of lenalidomide, and Henoch-Schönlein purpura. A total of 9% had no identifiable association.11
SR in acute lymphoblastic leukemia is a rare phenomenon. Among the 60 cases studied, only eight patients had ALL with SR. Table 1 resumes our case and all cases of ALL with SR reported in the literature. ALL with SR was observed in both male and female patients with equal prevalence and a median age of 32.25 years. We note that the duration of remission is very variable and can be few days (14 days) to several years (>8 years).
| N° | Year | First author | Age, years | Sex | FAB Subtype | Cytogenetics/Mutations | Duration of remission |
|---|---|---|---|---|---|---|---|
| 1 | 2000 | Takezako3 | 79 | Female | ALL-T | Not disclosed | 1 year |
| 2 | 2008 | Yoruk2 | 4 | Female | ALL-T | Not disclosed | 4 weeks |
| 3 | 2009 | Chen16 | 14 | Male | ALL-B | Normal | 14 days |
| 4 | 2014 | Purohit7 | 46 | Male | ALL-B | Normal | 9 weeks |
| 5 | 2017 | Eisa1 | 19 | Female | ALL-B | Normal | 2 months |
| 6 | 2018 | Höres18 | 31 | Female | ALL | 46XX, del(5)(q13;q22); ACSL6 deletion | >30 months* |
| 7 | 2021 | Lüke17 | 46 | Male | ALL-B | 45XY, dic(9;12)(p13,p13) | >34 months* |
| 8 | 2021 | Lüke17 | 19 | Male | ALL-B | Not disclosed | >8 years* |
| 9 | Our case | 40 | Male | ALL-T | Trisomy8 | 9 months | |
Here, we present the case of early T-cell precursor ALL diagnosed on cytological criteria and on Flow Cytometric Immunophenotyping of blasts in bone marrow. He did not start chemotherapy since a progressive decrease of the blast rate was noticed. During follow up and monitoring, the patient showed CBC recovery and the blastic infiltration seemed less important, evaluated at 4%. Therefore, no cytological aspect of acute leukemia was noted and cytogenetic remission was confirmed. Although the mechanisms of SR are not clear, they include infections.2 Indeed, an immune-mediated cause secondary to a previous severe infection is one of the most common proposals12 that may contribute to leukemia remission through excessive activation and production of pro-inflammatory cytokines, such as tumor necrosis factor (TNF) and interleukin 2 (IL-2). These cytokines, all of which have an anti-leukemic effect, increase the activity of T cells, macrophages and natural killer (NK) cells.13,14 A case of SR of adult T-cell leukemia/lymphoma (ATL) associated to pneumonia was reported.3
Other possible mechanisms of SR are hormonal factors, tumor necrosis, inhibition of angiogenesis, apoptosis, adverse effects of blood transfusions and elimination of carcinogenic substances. Especially the association between transfusion of different blood components and SR was reported.6 Indeed, a possible anti-leukemic effect could be observed especially in patients who have been treated with non-irradiated blood products. This effect may stimulate remission mediated by transfusion-associated graft-versus-host disease (TA-GVHD) and graft-versus-host leukemia (GVL) reaction.
Our case went through SR for nine months. Indeed, ETP-ALL is a different subtype of T-cell lymphoblastic leukemia, first identified in 2009, due to its unique immunophenotypic and genomic profile. The prognosis with these patients was poor in previous studies because they were susceptible to induction failure, with more frequent relapse/refracture disease.15 This may explain the relatively short time to relapse. Reports of spontaneous remissions of ALL have shown periods of temporary remission varying in duration from 14 days to 8 years.16,17 It should be noted that the longest periods of remission are generally with patients who had SR but also received therapy.
SR of ALL is an uncommon and transient phenomenon9 that is rarely described in the literature. Its mechanisms are not well elucidated; however, it is necessary to better understand the processes underlying SR as this could lead to new therapies for ALL. Also, there is a relevant question that requires careful consideration: “Is it necessary to implement early cytotoxic therapy during spontaneous remission in ALL, since the remissions are transient?”
Written informed consent for publication of their clinical details and clinical images was obtained from the patient.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the background of the case’s history and progression described in sufficient detail?
Partly
Are enough details provided of any physical examination and diagnostic tests, treatment given and outcomes?
Partly
Is sufficient discussion included of the importance of the findings and their relevance to future understanding of disease processes, diagnosis or treatment?
Partly
Is the case presented with sufficient detail to be useful for other practitioners?
Partly
References
1. Shimony S, Liu Y, Valtis YK, Paolino JD, et al.: Nelarabine combination therapy for relapsed or refractory T-cell acute lymphoblastic lymphoma/leukemia.Blood Adv. 2023; 7 (7): 1092-1102 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Childhood leukemia
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 30 Nov 22 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)