Keywords
Chromolaena odorata, mesenchymal stem cell, nerve cell, cell differentiation
This article is included in the Plant Science gateway.
This article is included in the Cell & Molecular Biology gateway.
Chromolaena odorata, mesenchymal stem cell, nerve cell, cell differentiation
We have made some corrections and revision on the revised version.
In Methods, we have changed the amount of ketamine and xylazine from ml to mg/kg within Housing and husbandry of animals subheading. We also provided the actual concentration of the drugs used in this study. We have corrected the concentration of the cells from 106 to 106 cells within subheading Mesenchymal stem cells exposure with C. odorata extract. We have provided the reference for enumeration of differentiation nerve cells within subheading Enumeration of differentiation nerve cells.
In Results section, we have added the information that the characterization of isolated MSCs according to the criteria by the International Society for Cellular Therapy (ISCT). We have provided the note on Table 2 that the number of cell numbers and the means based on measurements in 16 fields of view with 100x magnification. Figure 2 has been deleted since the data is repetitive with Table 2.
In Conclusion section, we have deleted the words of “containing flavonoids and alkaloids”.
See the authors' detailed response to the review by Arief Boediono
See the authors' detailed response to the review by Sharun Khan
Stem cells have received significant attention in the medical field because of their extended growth characteristics and ability to differentiate into other cell types. Neural stem cells (NSCs) can repair damaged brain cells by undergoing differentiation into nerve cells and glial cells.1 In adults, NSCs are found in the subventricular zone (SVZ) of the lateral ventricles and the sub-granular zone (SGZ) of the hippocampus. In the SVZ, the differentiation of NSCs into nerve cells occurs through migration along the rostral migration stream to the olfactory bulb. While in the SGZ, NSCs migrate to the granule cell layer.2 However, the capacity of NSCs to replace lost cells is limited and the regeneration of nerve cells in the mammalian brain, despite spontaneous regeneration, does not compensate for all lost nerve cells.3 As an alternative to NSCs, mesenchymal stem cells (MSCs) could be employed to repair damaged nerve cells. MSCs can differentiate into various mesenchymal cells comprising fibroblasts, chondrocytes, osteoblasts, myoblasts, and adipocytes.4
Bone marrow derived MSCs become the most potent stem cells for cell replacement because of their efficiency, high proliferative capacity, and immunological naivety. A suitable media is required as a habitat for nerve growth for the successful differentiation of MSCs into nerve cells. The media can be modified by adding fibroblast growth factor-2 (FGF-2), FGF-8, brain-derived neurotrophic factor (BDNF), or particular substrates.5 However, there are some challenges in the differentiation process of MSCs because of the low resistance of nerve cells during the culture process. To overcome this, an inducer is needed during the MSC differentiation process. Genetics, epigenetic, and chemical inducers have been used.6,7 Recently, researchers have utilized plant extracts to increase cell production by enhancing proliferation and protecting stem cells during the growing phase.8
Chromolaena odorata has pharmacological efficiency in wound healing by involving cell proliferation, inhibiting cell apoptosis, and contracting the collagen lattice.9 The plant extract contains flavonoids and alkaloids, which contribute to establishing proper growth of nerve cells, protecting the extracellular environment in the nervous system, inducing glial cell secretion of nerve growth factors, and protecting neurons from oxidative stress-induced apoptosis.10 In this study, we investigated the potential of C. odorata leaf extract as an inducer for MSC differentiation from bone marrow into nerve cells, where its expression was then detected using reverse transcription polymerase chain reaction (RT-PCR).
An in vitro experiment study was conducted to assess the potential of C. odorata leaf extract as an inducer for MSC differentiation into nerve cells. The MSCs were isolated from the bone marrow of a Mus musculus (the house mouse) and four concentrations of C. odorata extract were tested. Randomization and blinding of the animals were not required in this study. Two control groups (positive and negative) were used. The effect of C. odorata extract on MSC differentiation into nerve cells were measured after nine days of incubation.
Extraction was conducted using a maceration method with methanol as the solvent. First, C. odorata leaves were washed using water and sun-dried for three days. Dried leaves were then crushed into simplicial powder using a DF-15 grinder (CGOLDENWALL). Then 500 grams of simplicial powder were mixed with 1 liter of 96% methanol for five days, filtered and stored for two days at room temperature. The filtrate was evaporated using a BUCHI R-300 Rotary Evaporator (BÜCHI Labortechnik AG, Meierseggstrasse, Postfach, Germany) at 40°C with 80–90 rpm before the extract was kept in a container.
A stock culture medium of 1000 ml mDMEM was prepared by mixing 1 g of DMEM powder with aquadest and was homogenized. Then 0.37 g of NaHCO3, 100 μL of non-essential amino acids (Sigma-Aldrich Pte Ltd, Singapore), 100 μL of insulin transferrin selenium (Thermo Fisher Scientific, Carlsbad, CA, US), 100 μl of gentamicin (Sigma-Aldrich Pte Ltd, Singapore), and 10 mL of 10% fetal calf serum (Sigma-Aldrich Pte Ltd, Singapore) were added to the solution. The mixture was then sterilized using microfiltration with a diameter of 0.22 μm.11
The male M. musculus mice of the strain BALB/c (eight weeks of age) were acclimated and fed ad libitum with standardized feed for one week under laboratory conditions with a 12 h light-dark cycle, 60% of humidity with 23oC. To minimize the number of animals, six mice were used with assumption that the number of MSCs from one mouse was enough for one set of repetitions (i.e., with four repetitions in this study, two other mice were prepared for any unexpected event such as if the MSCs did not grow).
The animal facility was cleaned and disinfected regularly. During the acclimation the animal facility was kept quiet with controlled environmental conditions. If there were abnormal behaviors (apathy or increased aggression), the animals were excluded from the study and returned to the breeding center of the Faculty of Veterinary Medicine, Universitas Syiah Kuala. The mice were anesthetized with 100 mg/kg of ketamine (Troy Laboratories PTY Limited) and 10 mg/kg of xylazine (Troy Laboratories PTY Limited) and the animals were sacrificed using a cervical dislocation technique per protocol.12 It was ensured that all animals were dead before any autopsy was conducted. The autopsies were conducted by a certificated veterinarian.
The femur and tibia were removed from the mice and washed using FBS solution to remove muscle and fat tissue. Both ends of the bone were cut, then the marrow contents were removed using a syringe and stored in a culture dish containing FBS and NBCS. The bone marrow suspension was pipetted and centrifuged at 3,000 rpm for 10 min and then rinsed four times with FBS and once with mDMEM to remove as many single cells as possible.10 Before incubation, the number of cells (cells/mL) was counted using a hemocytometer.
A 1 mL of bone marrow suspension containing 1 × 106 cells was placed in a petri dish containing mDMEM medium and cultured in an incubator humidified with 5% CO2 at 37°C. After one day of incubation, the media was replaced and 10 μL of plant extract was added with a concentration of 0.7 mg/mL (group M1), 0.8 mg/mL (M2), 0.9 mg/mL (M3), and 1.0 mg/mL (M4) as treatments group and no extract was added as the negative control. The cells were incubated for nine days with media replacement every two days.11
Enumeration of differentiation nerve cells
The enumeration was conducted following the previous recommendation.13 Cells were observed under a CKX41 inverted microscope (Olympus Life Science, Tokyo, Japan) with 100x magnification in 16 fields of view. The enumeration was performed in three replications and the results averaged.
Nerve cell gene confirmation using reverse transcription polymerase chain reaction (RT-PCR)
The gene of nerve-like cells was detected using RT-PCR. The RNA was extracted using Z6011 ReliaPrep RNA Cell Miniprep System following the manufacturers’ protocol (Promega, Madison, WI, USA). Then 1 μL RNA was converted into a reverse transcription template cDNA using GoScript Reverse Transcriptase and random primer (Promega, Madison, WI, USA). The reaction condition was maintained at 25°C for 5 min, 37°C for 60 min, and 70°C for 15 min. The amplification of the cDNA was carried out using GoTaq qPCR Master Mix (Promega, Madison, WI, USA) and 2 μL forward and reverse primers (β-actin and β-tubulin 3). The detailed primer sequences are presented in Table 1.14 The PCR product was then analyzed on 1% agarose gel electrophoresis with TAE buffer run at 80 V for 60 min then visualized using UV light at 312 nm.
To compare the differentiation of the nerve cells among different doses of C. odorata, the data were analyzed using analysis of variance (ANOVA) followed by Duncan’s post hoc test with a significance level of 5%. All analyses were conducted using SPSS software version 20 (IBM SPSS, Chicago, IL, USA) (RRID:SCR_019096).
Ethical clearance was obtained from the Research Ethics Committee of the Faculty of Veterinary Medicine, Universitas Syiah Kuala (No 110/KEPH/VI/2021) - PT Bimana Indomedical (No.R.07-20-IR). All efforts were made to ameliorate any suffering of animals. Efforts were made to minimize the pain, suffering and distress experienced by the research animals. The animals were provided with appropriate housing with ad libitum feeding, the appropriate anesthesia was used to minimize pain before the animals were sacrificed and all procedures were conducted by a certificated veterinarian with the animal care training.
The leaf extract of C. odorata with a concentration of 0.8 mg/mL was the most optimum concentration for inducing cell growth with mean total cells of 147.67. Therefore, the morphology examination of differentiated MSCs into nerve-like cells was conducted using this optimal dose. The observations focused on differentiated nerve-like cells with cytoplasmic appendages. We characterized the isolated MSCs according to the criteria by the International Society for Cellular Therapy (ISCT).15,16
The cell's cytoplasm consisted of dendrites and axons, which indicated the cells were similar to nerve cells. Herein, it was observed that there were some variations of the nerve-like cells, such as nerve cells (neuroblast) with apolar (Figure 1A), bipolar (Figure 1B), and multipolar (Figure 1C) forms. Some undifferentiated MSCs (Figure 1D and E) were also observed.
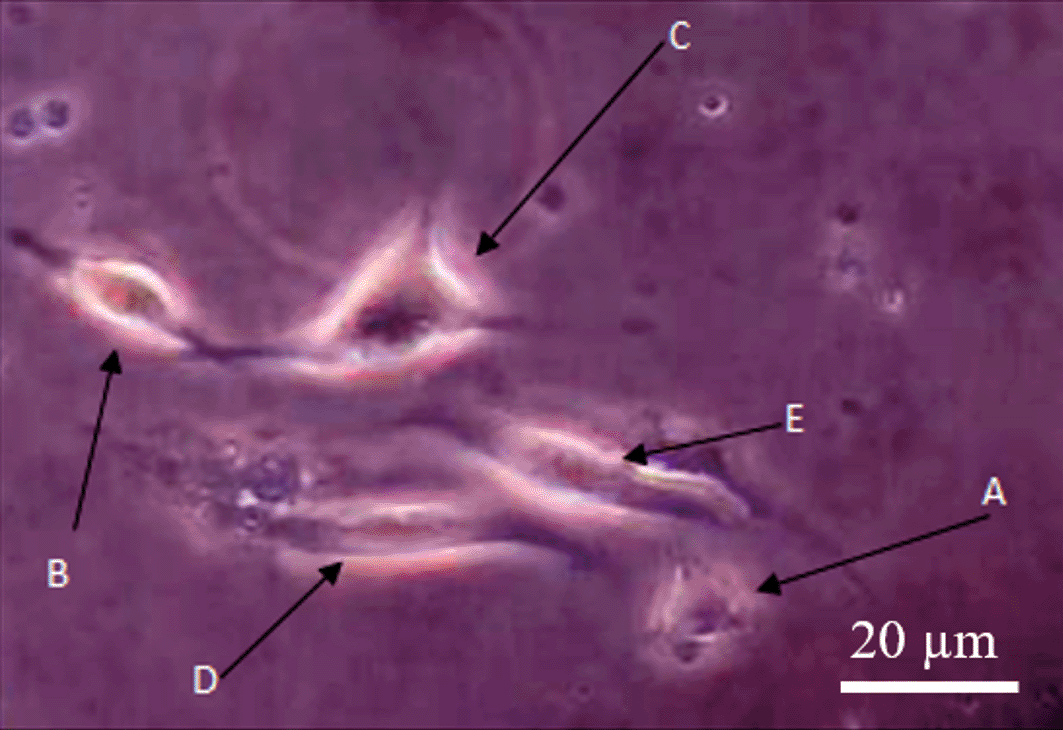
The differentiation process of nerve-like cells in medium culture showed the development steps of nerve-like cell formation, followed by dendrite extension and enlargement of the cytoplasm and dendritic bodies. Apolar nerve-like cells (Figure 1A) are the first development step of nerve cells, indicated by round and undeveloped extension of dendrites. The nerve-like cells then developed into bipolar form with two dendritic appendages (Figure 1B). The bipolar nerve-like cells then differentiated into multipolar forms characterized by multi dendritic extensions (Figure 1C).17 Bipolar nerve-like cells are classified as young nerve-like cells and multipolar as mature nerve-like cells.18
The mean number of nerve-like cells showed that the doses of C. odorata extracts had a significant implication on the differentiation (Table 2). The M2 group (0.8 mg/mL) had a higher number of differentiated nerve-like cells compared to other treatments (i.e., M1 (0.7 mg/mL), M3 (0.9 mg/mL), and M4 (1.0 mg/mL) and control groups. This suggested that 0.8 mg/mL was the most optimum concentration for inducing the differentiation of MSCs into nerve-like cells.
| Treatments | Cell numbers (Mean ± SD)a | Mean nerve-like cells (Mean ± SD) | Nerve-like cells (%) |
|---|---|---|---|
| Negative control | 86.00 ± 08.84 | 10.25 ± 02.98 | 11.9 |
| M1 0.7 mg/mL | 112.88 ± 15.7 | 19.00 ± 01.82* | 16.8 |
| M2 0.8 mg/mL | 147.67 ± 88.1 | 33.25 ± 0.957* | 22.6 |
| M3 0.9 mg/mL | 88.57 ± 13.6 | 14.25 ± 03.52* | 16.0 |
| M4 1.0 mg/mL | 102.48 ± 33.6 | 08.85 ± 02.64 | 8.6 |
Based on the statistical analysis, there was no significant difference between the control and M4 (1.0 mg/ml) group on inducing differentiated MSC into nerve cells. This indicated that 1.0 mg/mL had an inhibition effect on the cell culture. The decrease in the number of differentiated cells may be due to the use of excessive concentration. Extracts with excessive concentrations can cause cytotoxic effects on cells, so that cell growth becomes inhibited.19 Based on these results, the induction by C. odorata leaf extract on the differentiation of MSCs into nerve cells was better carried out at concentrations ranging from 0.7 to 0.9 mg/ml.
The leaf extract of C. odorata used in this study has active chemical compounds, such as flavonoids and alkaloids. Flavonoids are compounds that may also trigger nerve cell formation (neurodegeneration) by increasing the production of nitric oxide (NO). NO is used as a potent activator of the soluble guanyl cyclase enzyme. This enzyme is responsible for forming cyclic-guanosine monophosphate (cGMP).20 The concentration of cGMP can also decrease the conversion of cGMP into GMP by adding phosphodiesterase-5 (PDE-5), an inhibitor enzyme.21 The presence of NO might assist the conversion of cGMP into sildenafil. Sildenafil can lead to the increased protein expression of phosphatidylinositol 3-kinase (PI3K)-Akt. PI3K-Akt is one of the essential regulation factors for the neurodegeneration process and transmission of information to neuronal progenitor cells.22 Moreover, cGMP activates cGMP-phosphokinase G (PKG) pathways leading to the increasing cycle-adenosine monophosphate (cAMP) and element-binding protein (CREB) response that is essential for neuroblast viability.23 BDNF, cGMP, and P13K activity use Wnt signaling, which is the primary pathway in the differentiation process by increasing the number of receptors.24,25
A study found that alkaloids had an essential role in leading to neuroprotective effects by the inhibition of oxidative stress and the up regulation of BDNF expression.26 BDNF is one of the factors that can trigger the expression of nerve cells, which is essential in regulating plasticity, immune, and nerve formation (neuro-regeneration).27 BDNF can affect the survival and development of nerve cells by activating kinase receptor B enzyme in nerve cells and ganglia cells.28
In this study, the existence of nerve cells was (β-tubulin 3) confirmed using the RT-PCR. The control primer used in this study was β-actin. The β-actin primer was used to detect the actin gene, which is one of the housekeeping genes and the gene that is expressed within the cells in any tissue at any development stage of nerve cells.29 At the same time, β-tubulin 3 primer was used to confirm nerve cell gene expression.14 Figure 2 shows the DNA RT-PCR product of β-actin and β-tubulin.
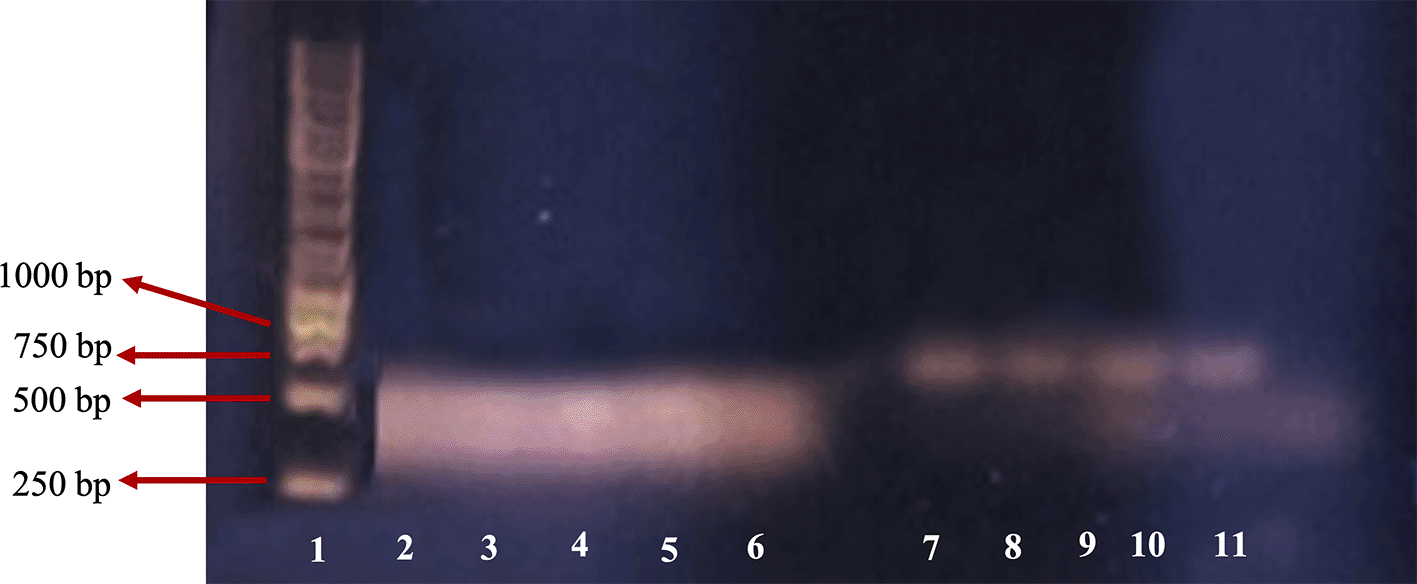
1: leaders; 2: β-actin of M4 group; 3: β-actin of M3 group; 4: β-actin of M2 group; 5: β-actin of M1 group; 6: β-actin of control; 7: β-tubulin 3 of M4 group; 8: β-tubulin 3 of M3 group; 9: β-tubulin 3 of M2 group; 10: β-tubulin 3 of M1 group; 11: β-tubulin 3 of negative control.
For the β-actin gene, PCR product was found in lanes two, three, four, five and six representing M4 (1.0 mg/mL), M3 (0.9 mg/mL), M2 (0.8 mg/mL), M1 (0.7 mg/mL), and the control group, respectively. DNA bands in lanes two, three, four and five are similar to those in the control (lane six). This indicated that RNA extraction was successful and no PCR inhibitor was found in the RNA solution extraction product.
For the β-tubulin 3 gene, there were positive DNA bands in lanes seven, eight, nine and 10 that represented M4 (1.0 mg/mL), M3 (0.9 mg/mL), M2 (0.8 mg/mL), and M1 group (0.7 mg/mL), respectively. Each band had clear and clean PCR product at the same size, while there was no PCR product in the negative control which suggests that the cells were nerve cells. The control band was not clear, perhaps due to a pipetting error. The size of the DNA band of the primer β-actin was about 443bp as expected, and the primer β-tubulin 3 was about 700bp, while according to Wang et al.,11 the size of targeted β-tubulin 3 was 55bp. This size difference was probably because the primer design was based on Rattus novergicus, the brown rat, while in this study we used M. musculus. Based on sequence data DNA similarity of the rat and mouse are high (90%), the primer also has potential for β-tubulin 3 detection in the mouse. However, confirmation of the PCR product with sequencing is required.
The application of C. odorata extract increased the differentiation of MSCs into nerve cells at the optimum concentration of 0.8 mg/ml. Identification with RT-PCR targeting β-actin and β-tubulin 3 confirmed the presence of nerve cell genes.
Figshare: Underlying data for ‘The differentiation of mesenchymal bone marrow stem cell into nerve cell induced by Chromolaena odorata extracts’. https://doi.org/10.6084/m9.figshare.19126544.30
This project contains the following underlying data:
Figshare: ARRIVE checklist for ‘The differentiation of mesenchymal bone marrow stem cell into nerve cell induced by Chromolaena odorata extracts’. https://doi.org/10.6084/m9.figshare.19126544.30
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cell culture, stem cell, embryo engineering
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Regenerative medicine, Stem cell therapy, Animal models
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cell culture, stem cell, embryo engineering
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Renesme L, Pierro M, Cobey K, Mital R, et al.: Definition and Characteristics of Mesenchymal Stromal Cells in Preclinical and Clinical Studies: A Scoping Review. Stem Cells Translational Medicine. 2022; 11 (1): 44-54 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Regenerative medicine, Stem cell therapy, Animal models
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 15 Jun 22 |
read | read |
|
Version 1 01 Mar 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
1) The figure 1 is not clear to conclude much information as stated.
2) The agarose gel for the lower base pair DNA amplification in figure 2 is very low. The agarose gel should be minimum 2% to get a clear picture for the amplification as the lane 2-6 looks like just primer dimers.
3) The authors have not provided any toxicity data of for the leaf extract. So it should be incorporated.
4) A positive control must be included in the article.
5) In Result and discussion paragraph 1 and 2, the authors have referred the figure 1 as individual Figure 1A, Figure 1B and so on. It should be only Figure 1 at the end of the sentence as individually it refers as there are 5 figures in Figure 1.
6) In method section's stem cell isolation paragraph, authors should write xg in place of 3000rpm along with the temperature of spin.
7) In RTPCR section it should be ng of primers and RNA in place of μl.
8) Show the neuron markers like TBR1 or TBR2 by imaging or flow.
1) The figure 1 is not clear to conclude much information as stated.
2) The agarose gel for the lower base pair DNA amplification in figure 2 is very low. The agarose gel should be minimum 2% to get a clear picture for the amplification as the lane 2-6 looks like just primer dimers.
3) The authors have not provided any toxicity data of for the leaf extract. So it should be incorporated.
4) A positive control must be included in the article.
5) In Result and discussion paragraph 1 and 2, the authors have referred the figure 1 as individual Figure 1A, Figure 1B and so on. It should be only Figure 1 at the end of the sentence as individually it refers as there are 5 figures in Figure 1.
6) In method section's stem cell isolation paragraph, authors should write xg in place of 3000rpm along with the temperature of spin.
7) In RTPCR section it should be ng of primers and RNA in place of μl.
8) Show the neuron markers like TBR1 or TBR2 by imaging or flow.