Keywords
p53, predictor, endocrine therapy resistance, luminal breast cancer
This article is included in the Oncology gateway.
p53, predictor, endocrine therapy resistance, luminal breast cancer
Firstly, we expanded the introduction with a tumor microenvironment in ET resistance and non-coding RNAs. Secondly, further explanation regarding several mechanisms of estrogen-unrelated genes in tamoxifen and fulvestrant-adapted cell line. Lastly, We did mention the co-expression of PGR and ESR1.
We also updated Figure 1.
See the authors' detailed response to the review by Norbert Nass
See the authors' detailed response to the review by Didik Setyo Heriyanto
Endocrine Therapy (ET) resistance in Luminal Breast Cancer (BC) is a concerning issue. Approximately 30-40% of Luminal BC are ET resistant, which leads to a higher recurrence rate and worsened prognosis. Although it has been extensively studied, till now there is no single predictive biomarker has been established to predict which patient will develop ET resistance during the 5-years-course of endocrine therapy.1–3
Such predictive biomarkers will be advantageous for clinicians and patients, as patients with a bigger chance of endocrine therapy resistance could be monitored closely. Perhaps later in the future, it could help to effectively change the course of the therapy before recurrence is established (and it becomes too late), as well as to help clinicians to identify which patients will not have ET benefits in the first place.1,2 As we know, the current trend in clinical trials of BC treatment is moving into personalized and tailored therapy for each case. Therefore, finding predictive biomarkers to predict ET resistance will also be critical for such therapeutic program development.3
Endocrine therapy resistance is a complex molecular process involving many development processes. Several hypotheses have been developed regarding addressing such a process and finding such predictive biomarkers. The resistance could develop at the start of the endocrine therapy (de novo or intrinsic resistance) or develop later during the endocrine therapy. The hypotheses range from the loss of hormonal receptor (HR) caused by ESR1 gene mutation and epigenetic mechanism,4–7 altered expression of co-factors (such as NF-kB, AIB1, SRC-1),8–10 crosstalk between ER and growth factors signaling (such as Her2neu, Insulin-like growth factor-1 receptor (IGF-1R))3,5,7,9,11,12 absent or reduced expression of a negative regulator such as p21 and p27,13–15 metabolic resistance caused by polymorphism or loss of CYP2D6 (main enzymes responsible for converting tamoxifen into its active metabolites),2,3,7,9,16,17 NF1 mutation lead to MAPK pathway activation,18–23 APOBEC mutation associated with PI3KCA mutation.24
The molecular mechanism of Estrogen Receptor (ER) and Progesterone Receptor (PR) actions are studied extensively for their association with ET resistance in Luminal BC. These molecular mechanisms additionally become an essential basis in rationalizing treatments such as Cyclin-CDK (Cyclin-Dependent Kinase) inhibitor and PI3K/Akt/mTOR inhibitor, which have been internationally accepted as current adjuvant treatments for Luminal BC with recurrence after ET resistance. Their actions, therefore, are fundamental knowledge to find a logical explanation of endocrine therapy resistance, and most of the hypotheses above could be explained by the disruption of the ER and PR mechanism of actions, resulting increased cellular proliferation and decreased apoptosis.3,5,7–15,25,26
p53 mutation is one of the most frequent genetic alterations in BC, found in approximately 28.3%-35% of overall BC patients, with higher incidence in Luminal B BC (30-55%), Her-2neu overexpression (70%) and TNBC group (80%).27–29 p53 mutation in positive hormonal BC will result in distinct poor prognosis, and especially seen in Luminal B BC with higher frequency and stronger association to poor prognosis compared to Luminal A BC.28,30 The mutation of this profound tumor suppressor gene may occur at the early onset of Luminal BC or progressively in the later course of the disease due to cancer cells’ ability to form more mutations in the advanced stage.19,27,31–33
p53 mutation has been known for more than four decades. Its extensive roles span cell cycle regulation, DNA repair, apoptosis process, cell metabolism, and immune response in the tumor microenvironment.20,33–37 This versatile tumor suppressor gene has been studied in many cancers, including breast cancer. Numerous endocrine resistance breast cancer studies conclusively found its protein accumulation and its mutation.19,31,32,38,39
This review will explore the current knowledge of ER and PR molecular mechanisms and their impact on initiating ET resistance in Luminal BC. Furthermore, we will discuss the apparent effect of p53 mutation on their molecular mechanisms, consequently aggravating ET resistance.
Estrogen is a steroid hormone in several tissues, such as the skin, liver, bone, and breast. Estrogen’s potent mitogenic effect in breast tissue will generate breast epithelial proliferation, alveolar growth, fat deposition, and fibrous tissue development during puberty, pregnancy, and lactation phases. These unprecedented changes in the breast are affected by Estrogen, which works alongside Progesterone and other growth factors.40
The active form of Estrogen in breast tissue, Estradiol, and its metabolites have been acknowledged as essential factors of early malignant transformation, such as DNA single-strand breaks and chromosomal impairment. Furthermore, it may lead to uncontrolled cell proliferation, accompanied by the development of cellular signaling collaborating in the cancerous cells’ progression. All events mentioned above will benefit the growth of cancer cells, and all depend on the molecular mechanism of ER in BC cells.41,42
Estrogen Receptor has a paramount role in BC cells, as described above. Hence it becomes the main target of endocrine therapy such as ovarian blockade, SERM (Selective Estrogen Receptor Modulator, i.e., Tamoxifen), and SERD (Selective Estrogen Receptor Degrader, i.e., Fulvestran).9,21
Being a nuclear receptor family member, ER-α and ER-β are the two different types of Estrogen Receptors. In breast tissue, the ER-α has a dominant role. Meanwhile, ER-β is still considered controversial and has an unclear role.43 Another estrogen receptor type is the G-coupled Estrogen Receptor (GPER), paramount for estrogen molecular action via the membranous mechanism.44
ER-α coded by the ESR-1 gene in chromosome 14, with an identical structure as other nuclear receptors, consists of 4 structural and functional domains. These domains are the amino-terminal domain (A/B domain), DNA binding domain/DBD, hinge region (D domain), and Ligand-Binding Domain/LBD.45
While estrogen binds with ER, the heat shock proteins (HSP70 and HSP90) will dissociate ER from this binding in the cytosol. This dissociation will cause conformation changes and form dimers, then ER-Estrogen dimers will be transported to the nucleus by D-domain, and subsequently the dimers will form attachment to EREs.9,25,46,47
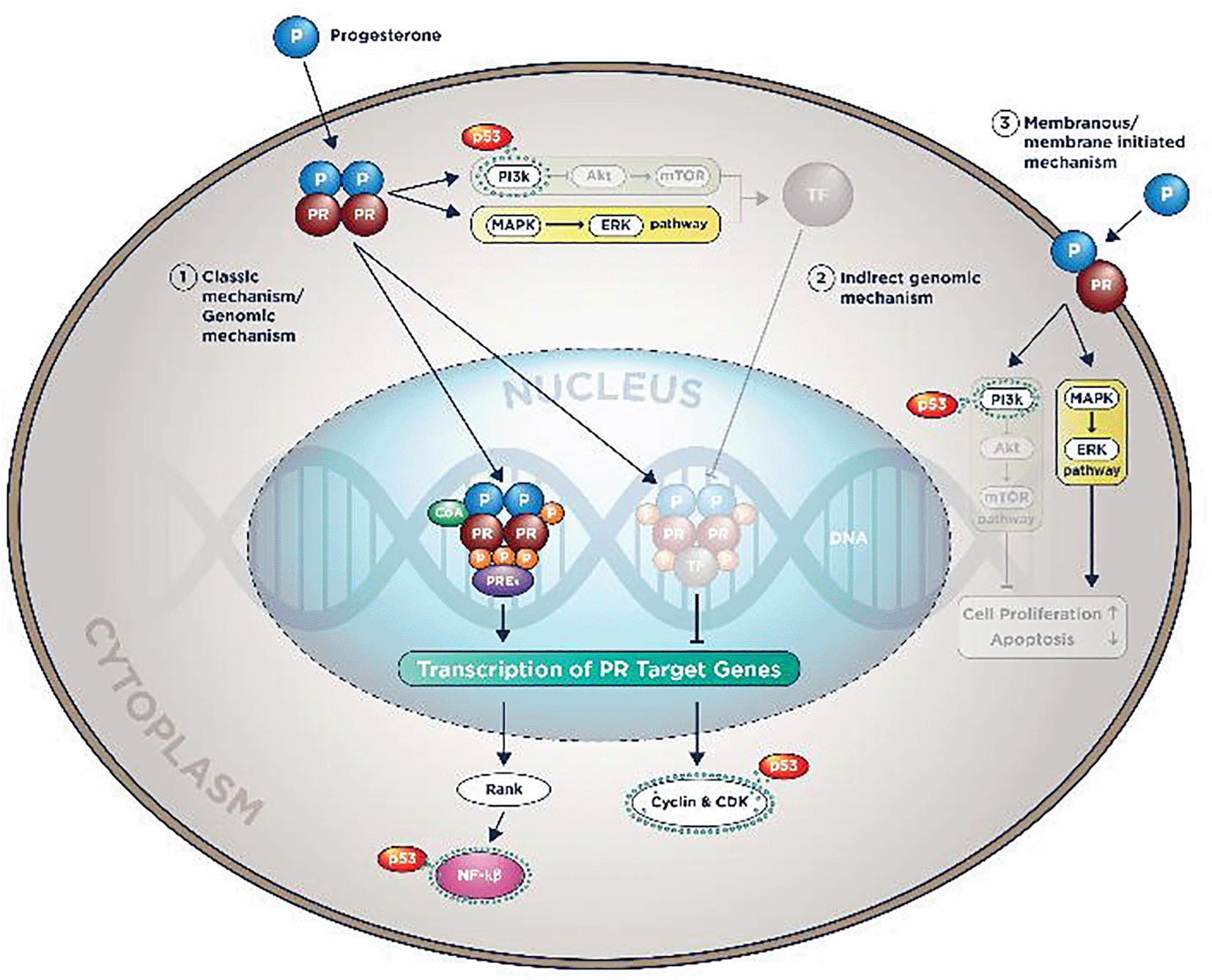
Subsequently, after entering the nucleus, DBD, with the aid of co-activators, will bind to Estrogen’s target genes that contain Estrogen Response Elements (EREs). The known co-activators are steroid receptor co-activator-1/SRC-1, SRC-2, and SRC-3 (AIB1/Amplified in Breast-Cancer 1). The binding of ER and EREs will activate the transcription of Estrogen’s target genes.3,48,49 This process is the so-called classic mechanism of ER molecular action, depicted in the figure below, along with other mechanisms. This mechanism is the first known ER molecular action and has become the theoretical basis for applying traditional endocrine therapy in luminal BC, such as ovarian blockade, SERM, and SERD.9,23
The estrogen receptor molecular actions are complicated and involve the intersecting apoptotic along with the survival pathways such as PI3K/Akt/mTOR, MAPK/ERK, resulting in the same similar target genes such as Cyclin-CDK, growth factor, and its activators.9,50 Currently, there are four known molecular mechanisms of ER actions: classical (genomic), non-classical, non-genomic (membranous), and ligand-independent (estrogen-independent). These four mechanisms are pictured in Figure 1.
In the non-classical mechanism (2nd mechanism in Figure 1), Estrogen could activate genes transcription that doesn’t contain EREs with help from tethering co-factors such as NF-kB (Nuclear Factor-Kappa Beta), activator protein 1 (AP-1), or specificity protein 1 (SP-1).3,9
In the membranous mechanism, Estrogen will not be required to enter the nucleus to do the genomic action since ER receptors conduct all the inciting processes in the cell membrane. In the last mechanism, even Estrogen is not required to induce its target genes' transcription (hence the name ligand-independent mechanism).3,9
These mechanisms will induce the exact effect on breast cancer cells: accentuating proliferative pathways and diminishing pro-apoptotic pathways. These non-classic, membranous, and particularly ligand-independent mechanisms make cancer cells more resistant to endocrine therapy. It is as if these estrogen receptors’ mechanism of action is being “hijacked” by the cells; the cancer cells are manipulating it to their benefit, that is, to replicate more and become less sensitive to apoptotic signals.4,9,21,44
The result of these molecular mechanisms of ER receptor actions are constant activation of the estrogen receptor target genes although there were no more estrogen molecules available ( i.e., due to ovarian blockade or inhibition by aromatase inhibitor), and although its receptor being blocked or degraded (i.e., due to inhibition by SERM/SERD).4,9,21,44 Despite this established ER receptor actions, we acknowledged that ET resistance is multifactorial and we cannot exclude several other non-estrogen related pathways in several studies using tamoxifen and fulvestrant adapted cell lines.51–54
Progesterone is a steroid hormone produced by the corpus luteum in the human ovarium, which its primary duty is to prepare the female body for gestation. Breast epithelial cells are indispensable in affecting duct-alveolar changes in phases such as puberty, the luteal phase (pre-menstrual period), pregnancy, and lactation.55
Compared to Estrogen, the role of Progesterone in breast cancer cells, especially endocrine therapy, and its resistance is less distinctive and less studied. The cyclic level of Progesterone and its hundreds of active metabolites available in the female body are the main difficulties in testing this hormone.56 PGR and ESR1 are usually co-expressed and only minor cases described PGR expression alone.57 Furthermore, the target genes of ER and PR overlap, thus adding to the complexity of this issue.56 Still, as epidemiological observations have shown, one cannot ignore the fact that the combination of Progesterone and Estrogen will add a mitogenic effect to BC cells in the animal model.58–60
PR was transcribed by three means. First, its transcription is induced by Estrogen as PGR (the gene for encoding the PR) is one of the Estrogen target genes. Estrogen has been proven to be required in maintaining PR levels in breast and endometrium epithelial cells.61 Second, cancer cells could induce PR transcription mediated by Insulin Growth Factor-1 (IGF-1) and MAPK/ERK activity. Even more, at high Progestin concentration, these growth factors will be re-induced and thus will re-activate the ER-α phosphorylation in the ligand-independent mechanism of ER (review above figure), resulting in more PR transcription.62
Some of the Progesterone target genes are also known to overlap with Estrogen target genes such as Cyclin-CDK, RANKL (Receptor activator of nuclear factor-kappa-Β ligand), and other growth factors. Hence these crosstalk mechanisms between ER and PR are crucial for breast cancer cell carcinogenesis and endocrine therapy resistance.4,62
Like Estrogen, the action of Progesterone in cells is entirely dependent on its receptors, and it consists of both nuclear and membranous receptor types. There are two types of nuclear PR: PR-A and PR-B. Both nuclear receptors exist in breast epithelial cells in variable amounts and activity. Furthermore, which nuclear receptor is more dominant in breast epithelial cells is unclear.56
Identical to ER, PR has an N-terminal domain, Ligand Binding Domain/LB, Progesterone will bind the PR and DNA Binding Domain/DBD in which target genes contain Progesterone Receptor Elements (PREs) will bind.63
After entering the cell’s cytoplasm, Progesterone will form a dimer, bind to PR in LBD, enter the nucleus, and bind to PREs with a co-factor. This process will activate the transcription of PR target genes. This is known as the PR action’s classical/direct genomic mechanism.56 Other mechanisms known are the non-classical/direct non-genomic and membranous mechanisms, depicted in Figure 2.15,56
In the indirect genomic mechanism, progesterone could activate genes that do not contain PREs as long there are tethering co-factors. In the membranous mechanism, PI3K/Akt/mTOR pathway and MAPK/ERK are also activated by progesterone. Therefore, it will accentuate the proliferative pathways and diminish the pro-apoptotic signals.15,56
The complicated cellular signaling regulates the cell cycle to maintain the regular cell proliferation rate and minimize errors in DNA synthesis. In this cell cycle, abnormal cells with DNA error will be ceased in G1-S transition critical point.64
Essentially, this critical G1-S transition point is determined by the interaction of Cyclin-D1& CDK4/6. This interaction will release E2F protein from its bond with Retinoblastoma Protein (RB Protein) in conditions without inhibition. The E2F protein will further trigger the cell to enter the S phase. Then consequently, abnormal cells with DNA will be duplicated.65
This mechanistic complex is one of the most often disrupted cellular signaling. It is found in endocrine therapy-resistant breast cancer cells, as Cyclin D1 (CCND1) and CDK4 become the target genes of Estrogen and Progesterone. Previously, Cyclin-CDK is still transcribed by the cancer cells, although the Estrogen production has been diminished and their receptors have been blocked.21,48,49 Relevantly, CDK 4/6 inhibitor has been approved in clinical guidelines as an adjunctive for endocrine therapy in Luminal BC, both pre-and postmenopausal patients.12,66
In normal cellular regulation, cells with abnormal DNA will be forced to enter the G0 phase by the p21 protein, a protein transcribed and regulated by p53. This p21 protein will inhibit the CyclinD1-CDK4/6 complex, resulting in the cell entering the G0 phase and starting the DNA repairing process. This well-regulated system earned p53 the old nickname: “guardian of the genome”.67,68
PI3K/Akt/mTOR pathway is a series of consecutive intracellular signaling that will activate proliferation and prevent apoptotic events. Genetic accumulation in this pathway and mutation of its inhibitor (PTEN/Phosphatase and TENsin homolog deleted on chromosome 10) are found in about 70% of the whole BC population.11,69
PI3K is an intracellular lipid kinase enzyme that will phosphorylate phosphatidylinositol molecule in the cell membrane, turning phosphatidylinositol-4,5-bisphosphate (PIP2) into phosphatidylinositol-3,4,5-trisphosphate (PIP3).69 Afterward, PIP3 will facilitate interaction between phosphoinositide-dependent kinase 1 (PDK-1) and Akt in the cell cytoplasm, resulting in a phosphorylated Akt. This phosphorylated Akt will activate Forkhead box O transcription factor (FoxO), which inhibits pro-apoptosis genes and activates mechanistic targets of rapamycin (mTOR) complexes.11
The mTOR complexes consist of 2 active forms: activated mTORC1 and mTORC2. mTORC1 will activate genes involved in carcinogeneses like protein synthesis, pro-survival genes, and cell growth. mTORC2 will specifically enhance phosphorylation, further causing Akt hyperactivation.11,69
In ER-α molecular actions, PI3K/Akt/mTOR will be activated in the non-classical, membranous, and ligand-independent mechanism.9,44,50,70 A likely, PI3K/Akt/mTOR will also be activated in PR molecular actions.15,56 Additionally, mTOR1 will activate S6K, which will help to phosphorylate RE-α, further activating the functional domain of RE-α. Likewise, the Akt activates the NF-kB that functions as a co-factor in the non-classical and membranous mechanism of ER-α molecular actions.20
It is well known that PTEN, a classical tumor suppressor gene, will reverse PIP3 to PIP2; hence the subsequent Akt/mTOR activation will not occur. Without PTEN, PI3K/Akt/mTOR pathway will be hyperactivated.71
The wild-type p53 protein will activate PTEN gene transcription. In cells with mutant p53, PTEN gene mRNA expression will be drastically reduced compared to cells with wild-type p53 status.72 Another seminal finding by Jung et al. 2018 in cell culture studies shows cells with PTEN loss will cause PI3K/Akt/mTOR hyperactivation, causing mTORC1 and mTORC2 enhancement. Both will phosphorylate and activate wild-type p53 protein, which causes p21 transcription. p21 protein will further induce cells to premature senescence condition.73
Although Luminal BC is considered ‘cold tumor’ due to its low immunogenicity characterized by the low count of Tumor Infiltrating Lymphocytes (TILs) and low Programmed Death Ligand-1 receptor, the roles of tumor microenvironment in ET resistance progression could not be ignored.74–79 Recent study by Gomez, et al. 2020 showed that exposure of ER+HER2-cells to continuous RANK pathway (a member of the tumor necrosis factor receptor (TNFR) superfamily) activation by exogenous RANKL (receptor activator of nuclear factor-kB ligand) both in vitro and in vivo, will cause downregulation of HR and increased resistance to hormone therapy.11 Sobral-leite, et al. 2019 depicted that activation of the PI3K pathway, in breast tumor cells was positively correlated with tumor-infiltrating FOXP3-positive lymphocytes which will bring poor prognosis for the patients.80 Another seminal study by Anurag, et al. 2020 identify that Luminal B BC have significantly high immunologic properties gene expressions correlated with endocrine resistant BC such as IDO1, PD1, LAG3 which will induce cytotoxic T-cell tolerance and down regulation of T-cell activation, and those three genes are targetable for immune-checkpoint inhibitor.76
The roles of p53 in BC tumor microenvironment is also noted, among which of the most discussed is p53 and its association with NF-kB. Nuclear Factor-kappa Beta (NFKB) is a transcription factor family consisting of 5 subtypes: p50, p52, p65 (RelA), RelB, and c-Rel. With a vast target gene involved in chronic inflammation and cellular proliferation, NF-kB is studied extensively in many cancers, including endocrine-resistant Luminal BC. In cell culture studies, treatment with NF-kB inhibitors will evoke endocrine therapy sensitivity and toxicity; therefore, it has not been tested on humans.8
NF-kB target genes considered instrumental in endocrine therapy resistance evolution are Cyclin D1, D2, D3 dan E, anti-apoptotic protein Bcl-2, MDM-2, and PDL-1 (Programmed Death Ligand-1).8,25,81 In ER molecular actions explained above; the NF-KB works as a co-factor in non-classical and membranous mechanisms so that Estrogen’s target genes are still transcribed although Estrogen has been blocked.8,78,81–84
The p53 and NF-kB have a negative association, for one cannot exist if the others are activated. The function is also contradictive; NF-kB will cause cell proliferation, be anti-apoptotic, and enhance chronic inflammation, whereas p53 will regulate the cell cycle and trigger pro-apoptotic events when needed.81,85
The antagonistic mechanisms are various. Some studies noted wild-type p53 protein act as the direct promoter inhibitor of NF-kB target genes, therefore inhibiting the transcription of the NF-kB target genes. Others stated they compete with each other to get transcription co-factor 300. Wild-type p53 protein will also be known to inhibit the IKK enzyme (Inhibitor of Kappa Kinase, an enzyme to activate the active form of NF-kB). Without IKK, NF-kB couldn’t enter the nucleus and induce its target genes transcription.35,81,85
TNF-α, the multi-functional mediator in inflammation and cell apoptosis, is also noted in Luminal BC for its role in enhancing cellular proliferation via NF-kB activation.86,87 The wild-type p53 was also recognized in turning off TNF-α induced NF-kB activation. This action is achieved by binding and blocking the work of Disabled homolog 2-interacting protein (DAB2IP), a protein that will activate TNF-α to trigger NF-kB activation.86
A summary of all p53 works in ER-α and PR molecular actions can be seen in Figures 3 and 4.

Plausible p53 wild-type roles in reducing endocrine therapy resistance by prohibiting ER-α molecular actions.
Plausible p53 wild-type roles in reducing endocrine therapy resistance by prohibiting PR molecular actions.
Distinguished studies also showed us that numerous studies of non-coding RNA especially miRNA play important roles in ET resistance, such as miRNA such as miRNA-1972, miRNA-375 and miRNA-221.52,88
However there are still no clear depiction of p53 roles in ET resistance caused by miRNA involvement. It is due to the complexity of the network of both parties, in which miRNA could regulate p53 level and function and vice versa mutated p53 could regulate the miRNA expression and modulate miRNA biology activities due to its gain-of-function properties.89
Although p53 is prominently correlated with poorer clinical features such as a high proliferation index and higher grade and stadium, its usage in Luminal BC is still arguably limited.90 One possible cause is that the presence of ER seems to suppress the p53 mutation itself.91 From the epidemiological point of view, p53 mutation is more frequently found in HER-2 enriched group and the Triple Negative BC group rather than Luminal BC. However, the p53 mutation, when found in Luminal BC, is not without importance. In fact study by Lee et al. 2013 from 7739 patients showed us that p53 mutation is correlated with a higher proliferative index such as Ki-67 in Luminal A BC, and when combined, they affect the long-term survival of the patients.92
Since it was found 40 years ago, the p53 protein has been published in numerous cancer studies using protein detection or genetic testing. These factors and the versatility of p53 function in cells make a test for p53 widely known and readily available in most laboratories. Therefore p53 is an ideal predictor to be chosen.34
p53 protein accumulation is easily detected by immunohistochemistry (IHC) as a surrogate marker of its mutation. However, p53 immunohistochemistry testing in BC could present and correlates either with or without favorable gene mutations tested, further affecting its capability as a biomarker in BC.91 It is an obstacle that is also frequently found in other cancers, such as ovarian and gastric cancer.93,94
No clear cut-off of p53 positivity in IHC assay also has been noted as the cause and affecting p53 usage as a predictive biomarker in BC.95 Study by Kikuchi et al. 2013 tried to address this cut-off issue found that when we set the cut-off of p53 immunoreactivity into ≥50%, then it could be helpful to predict clinical behavior in Luminal Breast Cancer, especially Luminal B type (p<0.0001).96 This finding is also confirmed by another epidemiological study of 7226 patients by Abubakar et al. in 2019.30
Furthermore, p53 protein accumulation has been a limitation as a predictor due to many p53 protein isoforms formed within the tissue. These isoforms are many, and each is said to have its roles in molecular effects in cancer cells.27,91,97,98 This limitation has been countered with the suggestion of genetic testing such as PAM50 or Mammaprint that would replace the p53 protein accumulation testing. Although it has been deemed more accurate than the IHC assay, genetic testing is expensive and not readily available in most laboratories, becoming the deterrent factor for choosing this testing in a clinical setting.99
Endocrine therapy is a very beneficial therapy for Luminal BC patients. Its usage will decrease the 15-year mortality rate to 30-40%; consequently, its resistance will pose patients with a dismal prognosis. As mentioned above, an established predictive biomarker will help clinicians to identify which patients will not have ET benefits in the first place. Therefore their adjuvant therapy should be changed to other modalities to reduce recurrence and increase the overall survival rate.2 In future perspective, a predictive biomarker that could anticipate ET is undoubtedly needed for developing personalized and tailored therapy for Luminal BC patients.2,26
Developing and planning studies to identify such biomarkers is not easy since the endocrine therapy resistance theories mentioned before are complex. Estrogen metabolism in premenopausal and postmenopausal women are also different; hence the endocrine therapy given is different; therefore, these groups cannot be investigated together.5,44,100,101 With the previous reasons mentioned, a meticulously planned study embedded in RCT with carefully chosen patients and prospective analysis probably is best to identify such biomarker, explained very well in the seminal study by Beelen et al.3
Additionally, p53 mutation could occur as early as the pre-carcinogenesis period, in the early stage of BC, and in late/metastatic disease.19 Consequently, it will be compulsory to test the p53 mutation along the course of the disease and observe whether it correlates with ET resistance later.
p53 is also known to have particular effects in each type of breast cancer (luminal A/B, with or without HER2 positive status) due to BC heterogeneity.102 Several studies have been made to address this issue and concluded that Luminal B breast cancer is the most probable BC group in which p53 mutation could be helpful as a predictive biomarker to predict ET resistance occurrence.92,102 This fact is also supported by epidemiological data that showed p53 mutation was found in higher in Luminal B BC compared to Luminal A BC.29,30,103
Another exciting development is Neoadjuvant Endocrine Therapy (NET), which is currently being studied in a clinical trial, and reportedly has advantages in downstaging and increasing Breast Conserving Therapy (BCT) success.104 In the future, NET could reduce hospital stays for Luminal BC patients and outreach the undertreated patients group. When patients cannot go to the hospital for various reasons, endocrine therapy could provide more accessible and comfortable neo-adjuvant treatment than chemotherapy or radiotherapy.104 Therefore, the need to find such a predictive biomarker becomes more pressing and indispensable, and we have to explore p53 mutation as a plausible biomarker.
p53 is an important biomarker to be considered an ideal candidate to anticipate ET resistance in the future. Its role within pathways involved in the ER and PR molecular mechanisms is paramount and cannot be ignored. Its limitation as a predictor could be countered using proper genetic testing rather than protein marker. Well-planned studies will be a prerequisite to concluding whether p53 is truly useful as a predictive biomarker for ET resistance in Luminal BC patients, especially the Luminal B group, with an adequate observation period.
FH worked on Conceptualization, Data Curation, Formal Analysis, Funding Acquisition, Investigation, Methodology, Resources, Software, Visualization, Writing – Original Draft Preparation. YA involved in Conceptualization, Data Curation, Formal Analysis, Supervision, Validation, Writing – Review & Editing. SS involved in Project Administration, Resources, Software, Supervision, Writing – Review & Editing. FH wrote the draft of the article, YA and SS helped with final manuscript preparation. BH involved in Conceptualization, Project Administration, Software, Supervision, Validation, Writing – Review & Editing. All figures and animations are original to this manuscript, composed by FH and approved by YA, SS and BH. All authors read and approved the final manuscript.
The authors would like to thank Ricarhdo Valentino Hanafi MD from Faculty of Medicine, Pelita Harapan University for his moral support and linguistic help in developing this review.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cell Biology, Tamoxifen resistance in breast cancer, protein biosynthesis
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cell Biology, Tamoxifen resistance in breast cancer, protein biosynthesis
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Yes
References
1. Gasco M, Shami S, Crook T: The p53 pathway in breast cancer.Breast Cancer Res. 2002; 4 (2): 70-6 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Airway, Cardiovascular, and Mediastinal Pathology, Gastrointestinal, Liver, and Gallbladder Pathology, General Pathology.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 30 Nov 22 |
read | |
|
Version 1 18 Mar 22 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)