Keywords
RNA dependent RNA polymerase, SARS-CoV-2 virus, COVID-19 treatments, molecular docking, molecular quantum similarity, chemical reactivity indices, density functional theory
This article is included in the Cell & Molecular Biology gateway.
This article is included in the Cheminformatics gateway.
This article is included in the Emerging Diseases and Outbreaks gateway.
This article is included in the Coronavirus (COVID-19) collection.
The severe acute respiratory syndrome coronavirus (SARS-CoV)-2 virus causes an infectious illness named coronavirus disease 2019 (COVID-19). SARS-CoV is a positive-sense single-stranded RNA virus from the Betacoronavirus genus. The SARS-CoV-2 RNA-dependent RNA polymerase (RdRp) has an important role in the viral life cycle and its active site is a very accessible region, thus a potential therapeutic approach may be to target this region to study the inhibition of viral replication. Various preexisting drugs have been proposed for the treatment of COVID-19 and the use of existing antiviral agents may reduce the time and cost of new drug discoveries, but the efficacy of these drugs is limited. Therefore, the aim of the present study was to evaluate a number of ligands used as SARS-CoV-2 virus inhibitors to determine the suitability of them for potential COVID-19 treatment.
In this study, we selected a series of ligands used as SARS-CoV-2 virus inhibitors such as: abacavir, acyclovir, amprenavir, ascorbic acid vitamin C, azithromycin, baloxavir, boceprevir, cholecalciferol vitamin D, cidofovir, edoxudine, emtricitabine, hydroxychloroquine and remdesivir. These ligands were analyzed using molecular docking, molecular quantum similarity, and chemical reactivity indices defined within a conceptual density functional theory framework.
The analysis of molecular quantum similarity indices on inhibitors showed a high number of differences from a structural point of view. However, they are quite similar in their electronic density, obtaining the highest values in the electronic similarity index. Global and local chemical reactivity indices were analyzed.
These studies allowed for the identification of the main stabilizing interactions using the crystal structure of SARS-CoV-2 RdRp. The molecular quantum similarity and chemical reactivity descriptors provide novel insights into these ligands that can be used in the design of new COVID-19 treatments.
RNA dependent RNA polymerase, SARS-CoV-2 virus, COVID-19 treatments, molecular docking, molecular quantum similarity, chemical reactivity indices, density functional theory
Revisions inserted:
fk+ not fk- is for nucleophilic attack.
Answer: Done
Euclidean distance of 16.8664 (Table 4); not 22.2556 (Emitri/Acyc).
Answer: Done
Softness S= 0.3916, not 1.2136; electrophilicity =1.2136, not 1.6253; seen in Table 5.
Answer: Done
See the authors' detailed response to the review by Yogesh Kumar
See the authors' detailed response to the review by Truong Tan Trung
The severe acute respiratory syndrome coronavirus (SARS-CoV)-2 virus causes an infectious illness named coronavirus disease 2019 (COVID-19). SARS-CoV is a positive-sense single-stranded RNA virus from the Betacoronavirus genus.1 Most RNA viruses require an RNA-dependent RNA polymerase (RdRp) for replication and transcription of the viral genome, thus making it essential for their survival, which is why it is a noteworthy topic to investigate. Its protein ranges from 240 to 450 kD and consists of a catalytic core and is a conserved protein within RNA viruses, and therefore it has been proposed as a potential option for the development of antiviral drugs.2
Numerous drugs are being studied to treat COVID-19. In the USA, the first antiviral drug to treat COVID-19 in adults and children aged 12 years and older was remdesivir (Veklury). For hospitalized COVID-19 patients, remdesivir that is injected intravenously may be prescribed (Mayo Clinic).
Molecular docking, molecular quantum similarity (MQS), and global and local reactivity indices were used in this study to evaluate remdesivir and other related compounds such as abacavir, acyclovir, amprenavir, ascorbic acid vitamin C, azithromycin, baloxavir, boceprevir, cholecalciferol vitamin D, cidofovir, edoxudine, emtricitabine and hydroxychloroquine to obtain novel insights into how the process of stabilization of these ligands at the active site in the receptor structure takes place. Previous studies have demonstrated positive responses with different levels of effectiveness for these aforementioned compounds in the treatment of COVID-19 (Mayo Clinic).
Besalú et al., introduced MQS 30 years ago to study the similarity of molecules.4 The density functional theory (DFT) framework is used to connect quantum mechanics and quantum chemistry utilizing molecular docking and chemical reactivity indices.5 This research is leading to advances in the discovery of new treatment alternatives for COVID-19 and determining whether approved drugs, such as remdesivir, interact with other potential ligands.
The importance of drug discovery for COVID-19 treatment cannot be overstated. Drug discovery plays a critical role in identifying and developing effective medications to combat the virus and its associated disease. Here are some key points highlighting its significance: Lack of Existing Treatments: When the COVID-19 pandemic emerged, there were no specific antiviral drugs approved for treating the disease. Drug discovery became essential to identify new compounds or repurpose existing drugs that could effectively target the virus and alleviate its symptoms. Reducing Mortality and Severity: Effective drugs can help reduce the severity of COVID-19 symptoms and decrease mortality rates. This is especially important for individuals who are at higher risk of severe illness, such as the elderly and those with pre-existing health conditions. Easing Healthcare Burden: An effective treatment can help alleviate the strain on healthcare systems by reducing the number of severe cases that require intensive care and hospitalization. This can also free up resources for other medical needs. Accelerating Recovery: Drugs that speed up recovery can help infected individuals return to their normal lives more quickly. This is crucial for both individuals and the economy. Combating Variants: The SARS-CoV-2 virus has shown the potential to mutate and give rise to new variants. Drug discovery efforts are important not only for the original virus but also for addressing emerging variants that might have different characteristics and responses to existing treatments. Global Public Health: COVID-19 is a global health crisis that requires a coordinated international response. The development of effective treatments can contribute to controlling the spread of the virus across borders and reducing its impact on a global scale. Learning for Future Pandemics: The knowledge gained from drug discovery efforts for COVID-19 can provide insights and strategies for responding to future pandemics. It highlights the importance of preparedness and rapid response in the face of emerging infectious diseases. Diversification of Treatment Options: Different individuals may respond differently to treatments due to various factors such as genetics, age, and underlying health conditions. Having a variety of treatment options available increases the likelihood of finding interventions that work for a broader range of patients. Economic Impact: The economic consequences of the pandemic are significant. Effective treatments can contribute to controlling the spread of the virus, allowing businesses and industries to recover more rapidly. Boosting Public Confidence: Successful drug discovery efforts can boost public confidence by demonstrating that medical and scientific communities are actively working to address the pandemic and improve public health.
In summary, drug discovery for COVID-19 treatment is of utmost importance for saving lives, reducing the burden on healthcare systems, and ultimately bringing an end to the pandemic. It requires collaborative efforts from researchers, clinicians, pharmaceutical companies, and governments to identify and develop effective therapies that can combat the virus and its effects.
System preparation
The receptor structure, for the docking analysis, was extracted from the crystal structure of the SARS-CoV-2 RdRp (Protein Data Bank (PDB accession number, 6M71), which was adjusted using the Schrödinger (RRID:SCR_014879) suite 2017-1 Protein preparation Wizard (RRID:SCR_016749) module. i) The hydrogen bond network (H-bond) was optimized, and the protein structure refined, ii) at physiological pH, protonation states were determined using the PropKa utility, iii) The Impact Refinement (Impref) module was used to run a molecular minimization with heavy atoms constrained to a low root mean square deviation (RMSD) from the initial coordinates.6–8
Moreover, the molecular structures of the compounds were sketched using Maestro Editor (Maestro, version 11.1, RRID:SCR_016748, Schrödinger, LLC). Then, 3D conformations were obtained with the LigPrep (RRID:SCR_016746) module, with ionization/tautomeric states predicted under physiological pH conditions with Epik (RRID:SCR_016745). Subsequently, energy minimization was used to comply with the Macro model using the OPLS2005 force field. The free alternative is AutoDock Vina (RRID:SCR_011958).
Molecular docking
Glide (RRID:SCR_000187)9,10 with default parameters and Standard Precision (SP) model were used for docking investigations. The docking grid was created using default settings, with the co-crystallized ligand in the center. For the van der Waals radii of the nonpolar protein atoms, a scaling factor of 0.8 was applied to facilitate the binding of larger ligands. Extra precision (XP) was also utilized to expand alternate receptor conformations appropriate for binding ligands with unusual orientations via induced fit docking (IFD); this method allows the protein to undergo side-chain and/or backbone movements upon ligand docking. The results were optimized using the best predictions made by the 20 ns molecular dynamics.
Molecular quantum similarity measure
A molecular quantum similarity measure (MQSM) amid two A and B systems, known as ZAB, compares two molecules using their respective density functions (DFs). Both DFs can be multiplied and integrated in terms of their electronic coordinates, which are then weighted using a predetermined positive operator Ω(r1,r2):11–13
The nature of the operator used in Equation 1 provides the information being compared between the two systems and determines the similarity measure. For instance, if the chosen operator is the Dirac delta function (an efficient approach for functions with high peak values, such as the electronic density), i.e., Ω(r1,r2)= δ(r1- r2). One of the first similarity metrics employed is the overlapping MQSM; another widely used alternative is the Coulomb operator, i.e., Ω(r1,r2)=|1- r2|-1, resulting in a Coulombic MQSM. Even if the two molecules are equivalent, a similarity measure can be employed, which is known as a self-similarity measure (ZAA for the case of molecule A).12
Given a group of N molecules, we can generate a similarity measure for each of them with regard to the other molecules in the group, including themselves. With this, a symmetric matrix can be constructed where the i-th column of the matrix is the collection of all similarity measures between the i-th molecule and each of the components of the group, including itself. Each vector (column of the matrix) is a discrete representation (in N dimensions) of the i-th structure. These sets of vectors are a set of chemical descriptors that do not simply express another set of molecular descriptors as is often done, but rather each descriptor has its own set of unique properties.12,13
i. It is universal in that it can be derived from any collection of molecules and any individual molecule within that group.
ii. It is impartial because there are no other possibilities available in the construction process than those given the density functions and similarity measurements involved.
MQSM similarity index manipulation and visualization techniques
The similarity measure obtained for the group is unique once we have chosen a group of study objects and the operator in Equation 1; however, these measures can be combined or transformed to gain a new class of additional terms, which can be called Quantum Similarity Indices (QSI).
There are varieties of possible manipulations of the MQSM that result in a variety of QSI definitions. The most common ones are the following and are used in this paper13–16:
Carbó's similarity index
Carbó's similarity index between two molecules I and J is constructed from Equation 2. Because this index is also known as the cosine similarity index, it corresponds to the cosine of the angle formed by the density functions when considered as vectors. For any pair of compared molecules, this Carbo QSI has a value between 0 and 1, depending on the similarity between the two molecules (when I = J, the index approaches 1).13–17
Quantum similarity using Euclidean distance
Taking into account the similarity of Equation 3:
It can be simplified to the so-called Euclidean distance index when k = x = 2 and can also be defined as follows:
This Equation 4 forms the distance index of infinite order.18
MQSM definition
Quantum similarity measurements are in accordance with psychological perception and the apparent principle of similarity: “the more similar two molecules, the more similar their properties are.” This statement necessitates the construction of a molecule-to-molecule comparison, and the comparison of their densities is used to derive a quantitative measure of the quantum similarity of two molecules. Generally, the MQSM can be defined as the integral computational measure between two density functions, where the density functions are multiplied and integrated for the electronic coordinates in the relevant domain.19–21
{r1, r2} are sets of electronic coordinates related to the corresponding wave function, and the operator Ω (r1,r2) is positively defined and supported on the electronic coordinates.22–26
Types of measurements in molecular quantum similarity
The types of measurements are mainly determined by the information required, the selection of supported operators and the form of MSQM.18–20
MQSM overlap considering Equation 2:
The distribution of Dirac's delta, Ω (r1, r2) = δ (r1, r2), is the most typical and intuitive choice for such a positively defined operator. These selections transform the broad definition of MQSM to compute the overlap MQSM that obtains measurements of the volume by both electronic density functions when they are superimposed.17–20
The Dirac delta distribution, Ω (r1, r2) = δ (r1, r2), is the most typical and intuitive choice for a positive definite operator. This choice transforms the broad definition of MQSM to compute the overlap MQSM.17–20 The MQSM calculates the degree of overlap between molecular densities using information about the electron “concentration” in the molecule16–21:
MQS coulomb considering Equation 2:
When the operator (Ω) is replaced with the Coulomb operator, , the coulomb MQS is generated, which defines the electrostatic Coulomb repulsion energy between two charge densities20,21:
The Coulomb operator affects the overlap density functions. When considering molecular density functions as an electron distribution in space, this equation is simply an extension of the Coulomb operator for a distribution of continuous charge, thus can be used as electrostatic potential descriptors in some instances. This operator is correlated to electrostatic interactions and obtains the measurement of electrostatic repulsion between electronic distributions.15–19
Euclidean distance index considering Equation 3:
Another major transformation that can be expressed in terms of the classical distance is:
Here is the distance between a and b, and k = 2 is the definition of distance. Subsequently, the Euclidean distance between A and B (two quantum objects) is defined by:17–21
Occasionally it is written as: , where DAB has values in the range of [0,∞} but for situations where the compared items are identical, it converges to zero17–21:
The norm of the differences of the density functions can be used in the definition Equation 9. The distance or dissimilarity index can be used to define the Euclidean distance index, which can also be represented as15–21:
Alignment method: Topo-Geometrical Superposition Algorithm (TGSA)
In this research, the TGSA23 approach has been used to align the data. Gironés proposed the TGSA, in addition to programming and implementing it. This method assumes that the best way to align molecules is to superimpose them on a typical skeleton, with only the type atoms and the interatomic bonding interactions. The program accomplishes its purpose by examining the pairs of molecules and aligning the common substructure for a group.23 Only topological and geometrical considerations are used in the procedure, including molecular topology and how distant bonds are compared. The superposition of two molecules is unique and is independent of the type of operator used to determine the similarity.23,24
Molecular alignment is a critical factor in molecular quantum similarity studies, which aim to compare and analyze the similarity of molecules based on their quantum mechanical properties. Molecular alignment refers to the arrangement or orientation of molecules in a specific way that facilitates the comparison of their electronic structure and other quantum properties. The importance of molecular alignment in molecular quantum similarity can be understood through the following points: Consistent Geometries: Molecular alignment ensures that the molecules being compared are in consistent geometries or orientations. This is crucial because the electronic structure and quantum properties of molecules can vary significantly based on their spatial arrangement. By aligning molecules in a specific way, researchers can focus on comparing their intrinsic quantum properties rather than being confounded by geometric differences. Accurate Comparison: Quantum similarity aims to quantify the resemblance of molecular properties, such as electronic distributions and energy levels. Proper alignment enables a more accurate comparison of these properties across different molecules. It allows researchers to assess whether molecules with similar alignments also exhibit similar quantum behavior, providing insights into their functional and structural relationships. Quantitative Analysis: Molecular quantum similarity often involves complex computational methods to calculate molecular properties like electronic densities, molecular orbitals, and energy levels. Accurate alignment ensures that these calculations are based on consistent molecular arrangements, leading to reliable quantitative analyses of similarity. Drug Discovery and Materials Design: Molecular quantum similarity is employed in drug discovery and materials design to identify compounds with similar quantum properties to known active compounds or materials. Proper molecular alignment ensures that the comparison is meaningful, leading to the identification of candidates with potential similar functional properties. Structure-Property Relationships: Understanding how molecular alignment affects quantum similarity can provide insights into the relationship between molecular structure and properties. Researchers can gain a deeper understanding of how specific arrangements influence quantum properties and functional behavior. Enhancing Predictive Models: Machine learning and computational methods often rely on molecular quantum similarity to predict various properties of molecules. Accurate molecular alignment contributes to the quality of training data and helps in building more reliable predictive models. Quantum Chemistry Interpretations: Proper molecular alignment facilitates the interpretation of quantum chemical calculations. It helps researchers visualize and understand the impact of different molecular orientations on electronic structure, energy levels, and other quantum properties.
In summary, molecular alignment is crucial in molecular quantum similarity studies because it allows for consistent and accurate comparisons of quantum properties across different molecules. This alignment ensures that the observed similarities are not confounded by variations in molecular orientation, leading to meaningful insights into the functional and structural relationships between molecules.
Our research group has used several reactivity indices with Quantum Similarity.26–35 The reactivity indices used in this work are chemical potential (μ),36–38 hardness (ɳ),39 and electrophilicity (ω),38,39 which will be calculated.
The electrophilicity index (ω) measures the stabilization energy of the system when it is saturated by electrons from the external environment, and is mathematically defined as38,39:
In this study, the local reactivity descriptors are the Fukui functions. Equations 15 and 16 represent the response of the chemical potential of a system to changes in the external potential. It is defined as the derivative of the electronic density with regard to the number of electrons at the constant external potential:
Where is for nucleophilic attack and for the electrophilic attack.40–42 In this approach, using the global and local reactivity descriptors makes it possible to compare the molecular reactivity at the sample set. All the structures were developed using M02X/6–31G(d, p)43 methods in Gaussian 09 package.44
In this work, the stabilization process of a set of ligands related with activity against the novel SARS-CoV-2 was studied to understand the main ligand interactions in the active site, starting from the PDB crystal structure of SARS-CoV-2-dependent RNA polymerase code: 6M71.45,46 The crystal structure of SARS-CoV-2 RdRp was selected taking into account the outcomes reported by Elfiky et al.47 Figure 151 shows the docking interactions using remdesivir. In the conformation of Figure 1A and B, the docking interaction for the structure of remdesivir with a higher RMSD shows an -H bond with ARG555, ARG553 with two lengths of 2.28 Å and a length of 2.35 Å, LYS621 with a length of 2.22 Å, CYS622 with a length of 2.42 Å, and ASN691 with a length of 2.38 Å.
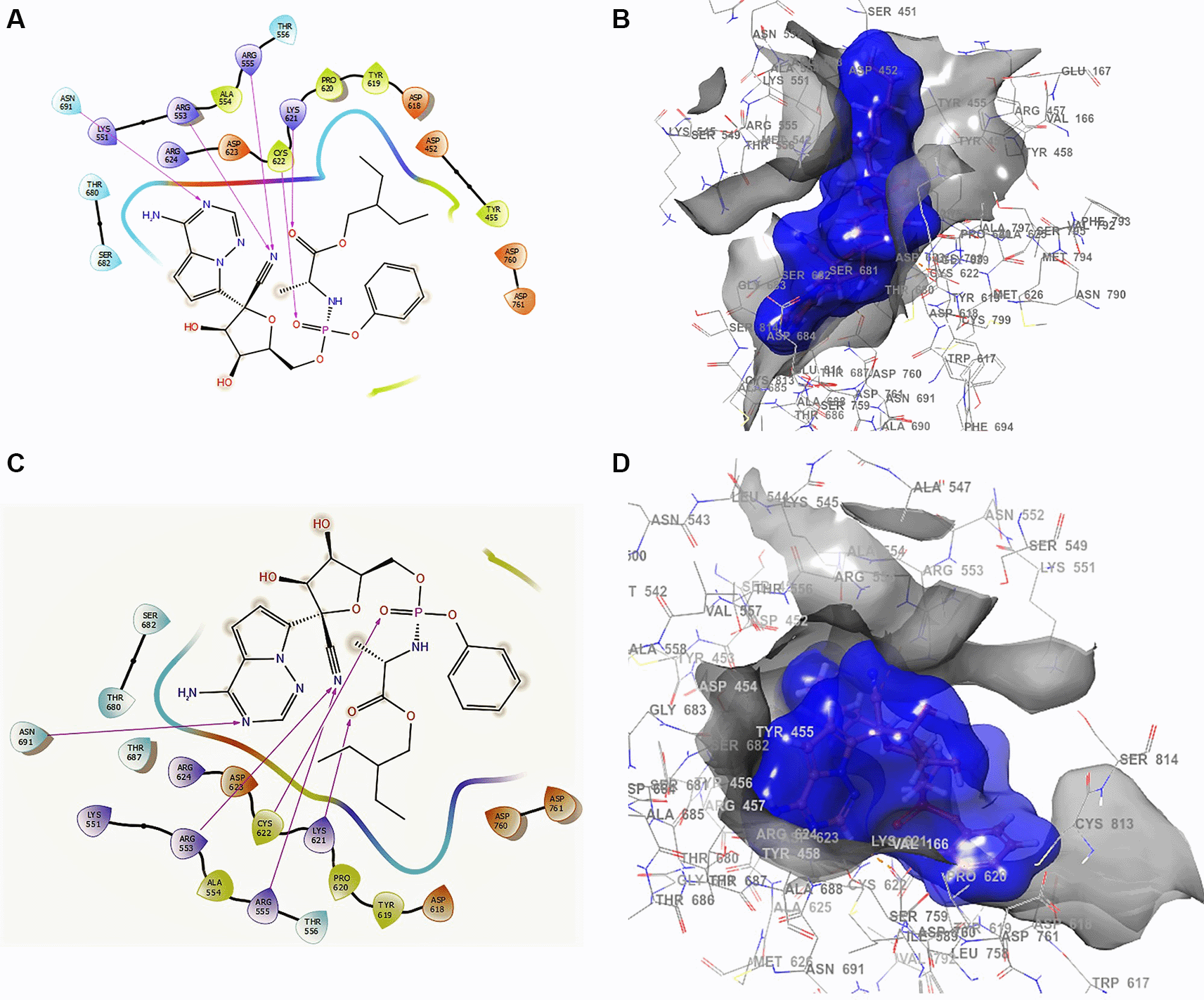
A. Docking interactions using remdesivir with the crystal structure of SARS-CoV-2 RdRp (PDB accession number 6M71). B. Docking interactions using Receptor (gray) and ligand (blue) surfaces. C. Docking interactions with the receptor site. D. Surface of the binding pocket with the receptor site. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RdRp, RNA-dependent RNA polymerase; PDB, Protein Data Bank.
Another of the best conformations of remdesivir (Figure 1C and D) involved a -H bond with residue ASN691 with a length of 2.46Å, two ARG553 and ARG555 with lengths of 2.35Å and 2.38Å, CYS622 with a length of 2.41Å, and LYS621 with a length of 2.39Å. The interactions with the residue ARG553 and ARG555 and LYS621 were very similar to Figure 1A and B. These interactions play an important role in the interaction of the active site, generating a good bonding surface (see Figure 1B and D).
Figure 2 shows the docking interactions using ascorbic acid vitamin C structure of SARS-CoV-2 RdRp from SARS-CoV-2. The ligand and receptor surfaces show the active site zone defined by the stabilization interactions. A phosphate group was found to reside well inside a local binding pocket within the grip with residue CYS622.
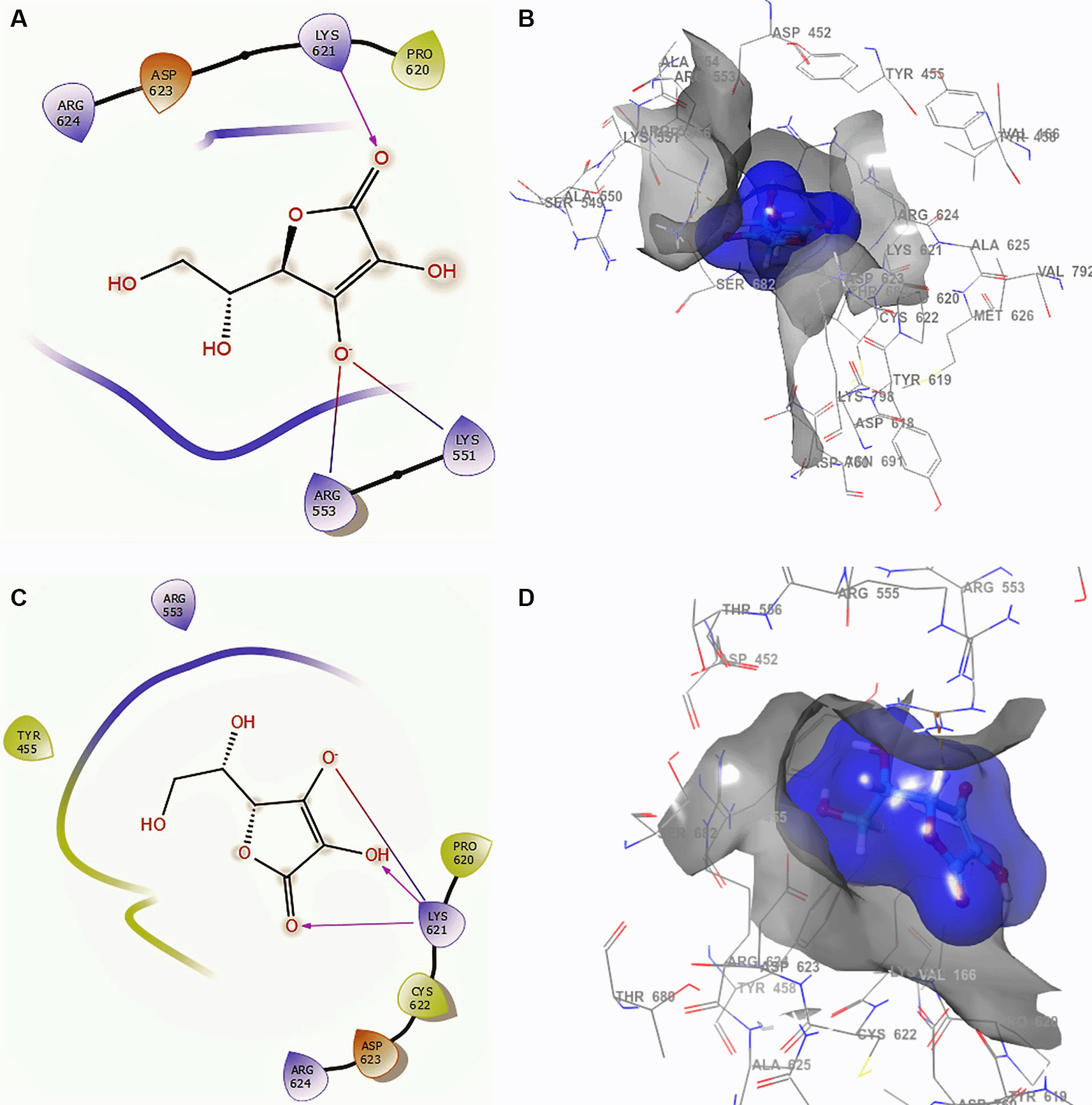
A. Docking interactions using ascorbic acid vitamin C with the crystal structure of SARS-CoV-2 RdRp (PDB accession number 6M71). B. Receptor (gray) and ligand (blue) surfaces. C. Docking interactions with the receptor site. D. Surface of the binding pocket with the receptor site. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RdRp, RNA-dependent RNA polymerase; PDB, Protein Data Bank.
In Figure 2A and B, the ascorbic acid vitamin C has -H bonds with the residues LYS621 at a distance of 2.20Å, ARG553 at a distance of 1.86Å, and LYS551 at a distance of 1.86Å, respectively. The ligand has the same interaction as remdesivir with residue LYS621. This conformation showed a good stabilization in the pocket, generating a good contour surface (see blue surface). Conformation 2 (Figure 2C and D) shows three -H bonds with the same residue LYS621 at distances 2.18Å, 2.25Å and 2.27Å. This residue LYS621 is also shown in the conformations of remdesivir.
Conformation 1 of Figure 3A and B shows the structure of cholecalciferol vitamin D, the largest RMSD corresponding to an -H bond with the residue SER759 at a length of 2.24Å. For conformation 2 in Figure 3C and D the -H bond is with the residue GLU166 with a length of 2.28Å. Finally, the compound has interactions with the residue TRP617 with a length 2.26 Å. Although this compound only interacted with the residue SER759, the ligand surface is well defined near the receptor, even though it is a single -H bond. The interaction is good enough to generate an active conformation with stabilizing capacity (Figure 3C and D), in agreement with previous reported results.48
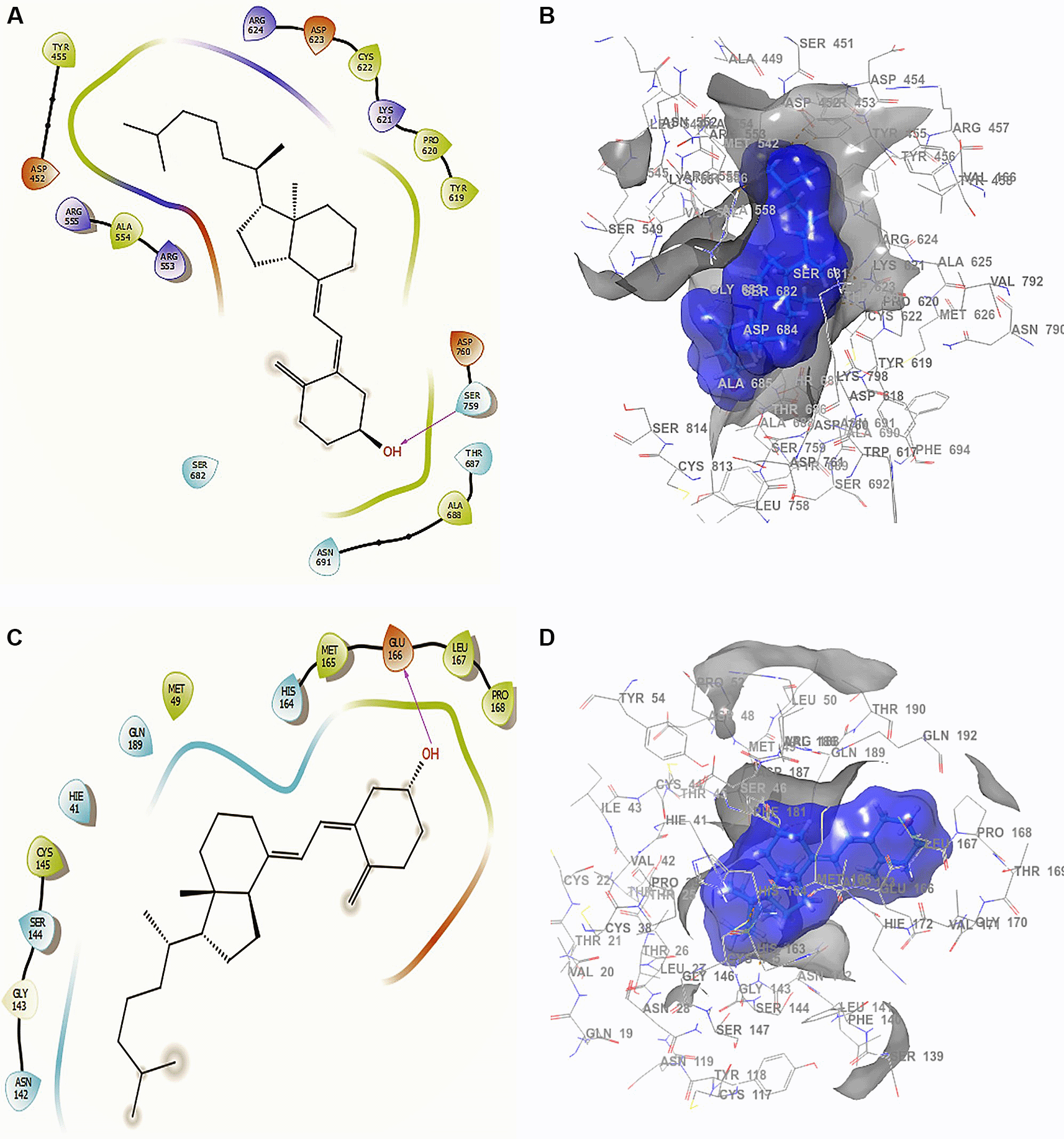
A. Docking interactions of cholecalciferol vitamin D with the crystal structure of SARS-CoV-2 RdRp (PDB accession number 6M71). B. Receptor (gray) and ligand (blue) surfaces. C. Docking interactions with the receptor site. D. Surface of the binding pocket with the receptor site. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RdRp, RNA-dependent RNA polymerase; PDB, Protein Data Bank.
Conformation 1 of azithromycin (Figure 4A and B), like those of remdesivir and ascorbic acid vitamin C, has -H bond with LYS621 with a length of 2.28Å. In addition, it has two interactions with the residue ASP760 at lengths of 2.26Å and 2.34Å, with ASP761 at a length of 2.32Å. On the other hand, for conformation 2 of azithromycin of Figure 4C and D, we can see two -H bonds with the residues ASP760 with lengths of 2.41Å and 2.38Å, ASP761 with a length of 2.38Å and TRP617 with a length of 2.28Å. These ASP760, ASP761 and TRP617 interactions agree with the recent work published by Abdrabbo et al.49 In this work, once the stable binding mode was located, free energy perturbation (FEP) calculations were carried out to estimate the binding affinity of RemTP and ATP to COVID-19, and to identify the key residues in the binding process.
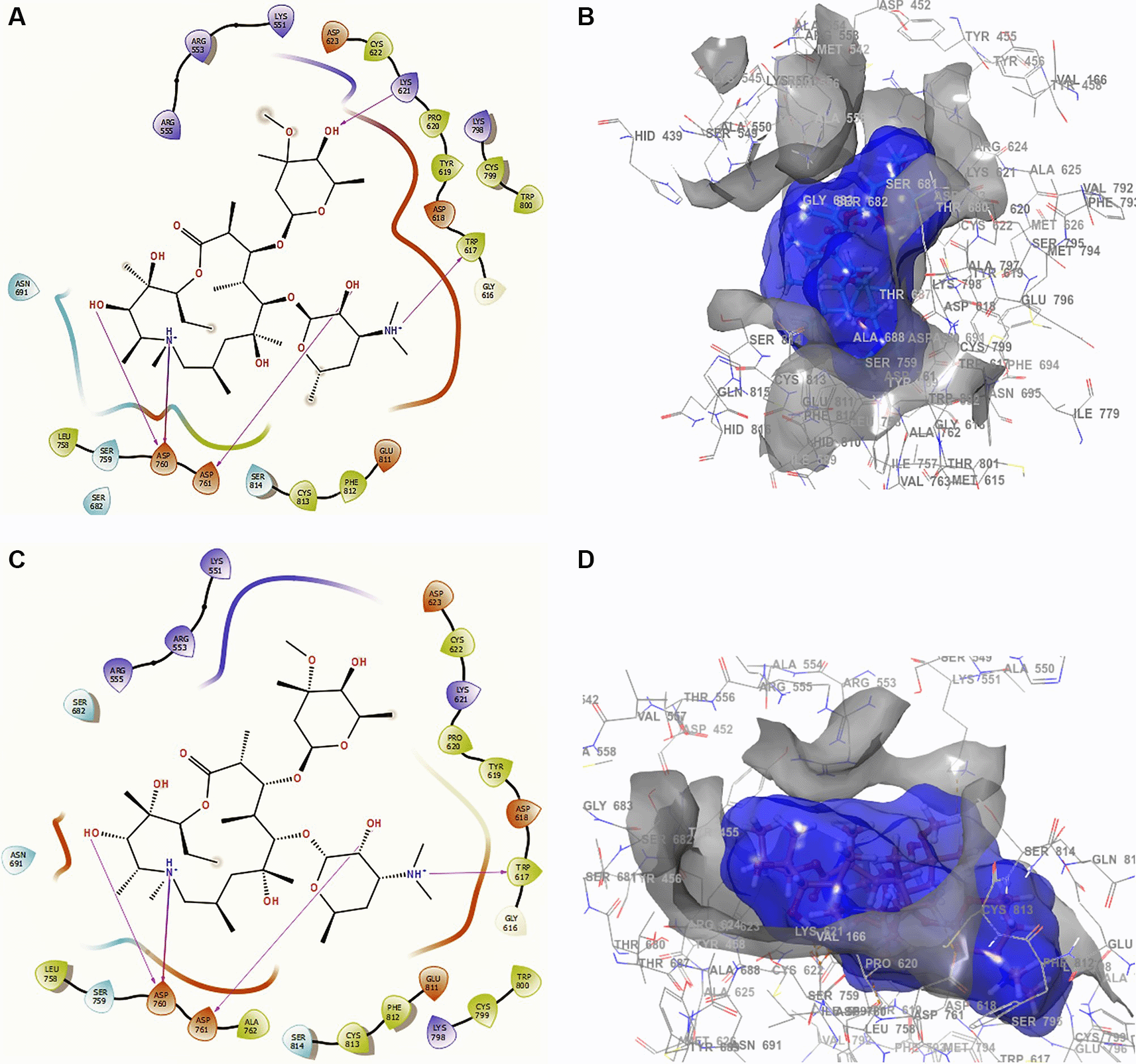
A. Docking interactions using azithromycin with the crystal structure of SARS-CoV-2 (PDB accession number 6M71). B. Receptor (gray) and ligand (blue) surfaces with a receptor (gray) and ligand (blue) surfaces. C. Docking interactions with the receptor site. D. Surface of the binding pocket with the receptor site. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RdRp, RNA-dependent RNA polymerase; PDB, Protein Data Bank.
The docking interactions in conformation 1 of Figure 5A and B for hydroxychloroquine with higher RMSD shows a -H bond with the residue ARG553 with a length of 2.38Å, with ASP760 at lengths of 2.36 Å, 2.28 Å and 2.32 Å. For the best conformation 2, Figure 5C and D involved ASP462, ASP623 and ASP760 with lengths of 2.36 Å, 2.31 Å and 2.28 Å, respectively.
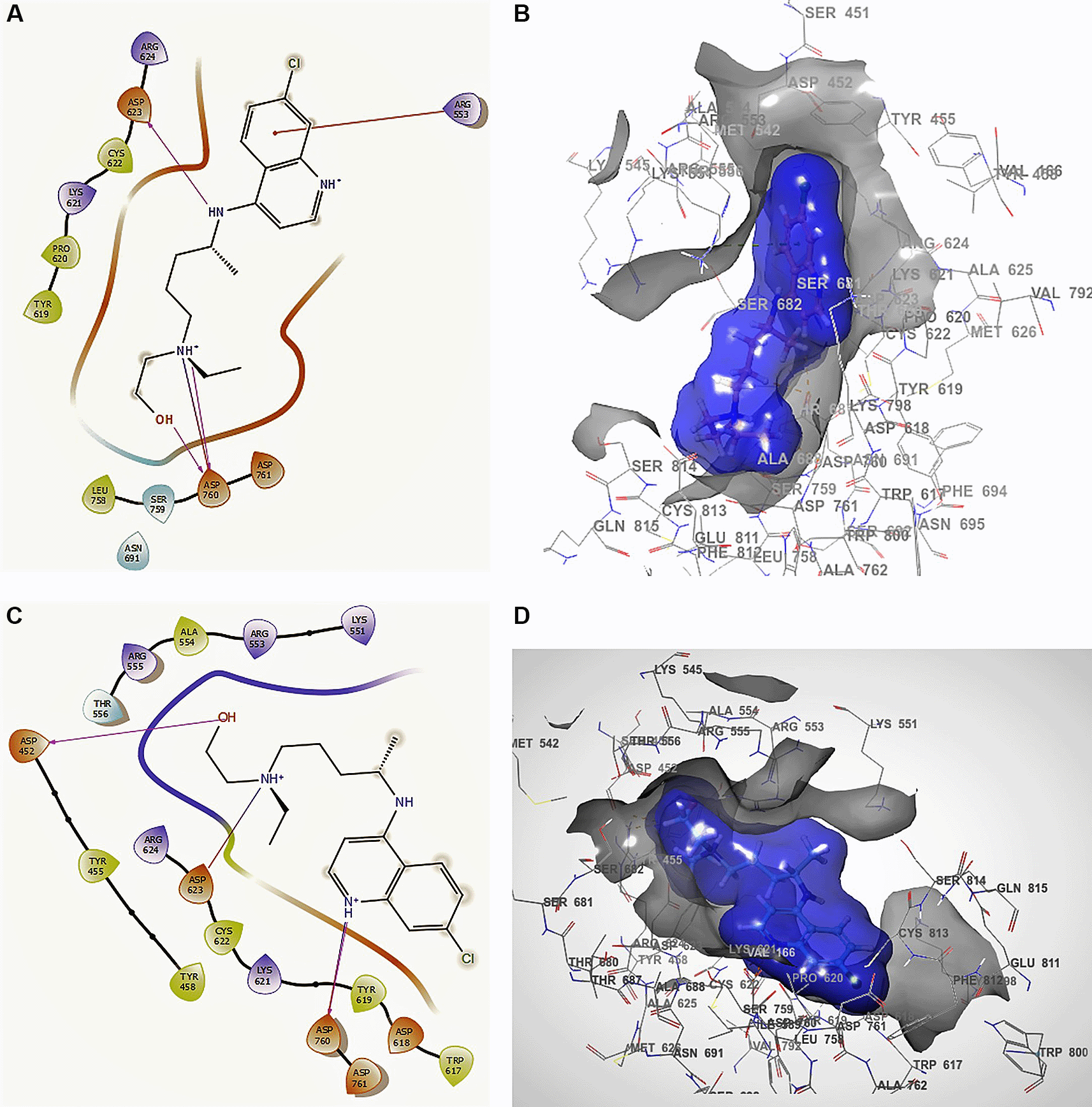
A. Docking interactions using hydroxychloroquine with the crystal structure of SARS-CoV-2 RdRp (PDB accession number 6M71). B. Receptor (gray) and ligand (blue) surfaces with a receptor (gray) and ligand (blue) surfaces. C. Docking interactions with the receptor site. D. Surface of the binding pocket with the receptor site. SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RdRp, RNA-dependent RNA polymerase; PDB, Protein Data Bank.
In this study, a molecular quantum similarity study was developed to systematically investigate and analyze the structural and electronic considerations involved in active site stabilization. An electronic similarity study has been designed to find the relationship between ligands and the active site of RdRp proteins.
Because conformations are crucial in docking studies, we have analyzed the impact that molecular alignment has on the generation of interactions (-H bond) at the active site. These molecular alignments, which generated the best poses, were related to potential COVID-19 inhibitors. For this analysis we have used four similarity descriptors (structural, electronic and their Euclidean distances) and molecular quantum similarity indices were calculated using the overlap and coulomb descriptors (Equations 6 and 7).
The compounds analyzed in the docking results were compared with a series of ligands with SARS-CoV-2 activity such as abacavir, acyclovir, amprenavir, ascorbic acid vitamin C, azithromycin, baloxavir, boceprevir, cholecalciferol vitamin D, cidofovir, edoxudine, emtricitabine, hydroxychloroquine and remdesivir. These compounds have been associated with COVID-19 treatment.
The molecular quantum similarity indices are defined on the interval (0,1), where 0 means dis-similarity and 1 means self-similarity. Table 1 shows the molecular quantum similarity indices using the overlap descriptor. Based on the distribution of Dirac's delta, Ω (r1, r2) = δ (r1, r2), this operator can be related to the structural characteristics of the compound analyzed. The highest values were for abacavir/acyclovir (0.6286) with an Euclidean distance of 3.5612 (Table 2), emtricitabine/abacavir (0.6011) with an Euclidean distance of 3.7645 and emtricitabine/edoxudine (0.6079) with a Euclidean distance of 3.8020. The lowest values were for boceprevir/azithromycin (0.1374), and remdesivir/cholecalciferol (0.1476) with a Euclidean distance of 7.1944.
The molecular quantum similarity indices using the Coulomb descriptor were calculated to obtain insights into the electronic similarity (see Table 3). The Coulomb descriptor is related to the electronic similarity in the molecular set. Table 3 shows that the highest values for emtricitabine/edoxudine (0.9390) with an Euclidean distance of 14.4795 (see Table 4), emtricitabine/abacavir (0.9311) with an Euclidean distance of (16.2459) and edoxudine/acyclovir (0.9165) with a Euclidean distance of 22.2556. Unlike the overlap descriptor, the values are near to 1. This means that despite having significant structural differences, these structures have high electronic similarities.
This work has also explored the global and local chemical reactivity indices within the DFT framework. Table 5 shows that the least reactive molecule was hydroxychloroquine, whose electronic chemical potential μ=-1.7070 eV, chemical hardness η=1.606 eV, softness S=0.6226 eV and electrophilicity ω=0.4536 eV. The most reactive compound was baloxavir-marboxil hydroxychloroquine, whose electronic chemical potential μ=-3.7335 eV, chemical hardness η=2.1441 eV, softness S=0.4664 eV and electrophilicity ω=1.6253 eV. These electrophilicity values play an essential role in ligands stabilized by non-covalent interactions and determine the stability of the active site.
The compounds with the highest chemical potential (negative electronegativity) were hydroxychloroquine with μ=-1.7070 eV, abacavir with μ=-2.6330 eV and cholecalciferol vitamin D with μ=-2.9825 eV. These compounds are commonly used in COVID-19 treatment.48–50 On the other hand, abacavir is used to treat HIV infection and some studies have shown its relationship with COVID-19.50 However, some countries are skeptical about its use for COVID-19 treatment. Other important compounds often used for the treatment of SARS-CoV-2 virus are azithromycin and remdesivir, with a chemical reactivity of μ=-3.5621 eV and -3.6097 eV, chemical hardness η=2.5532 eV and 2.3253 eV, softness S=0.3916 eV and 0.4300 eV and electrophilicity ω=1.2136 eV and 1.4009 eV, respectively. Both are very reactive and have high electrophilicity values. Figure 6 shows the Fukui functions that have been used to describe the local chemical reactivity of remdesivir and cholecalciferol vitamin D.
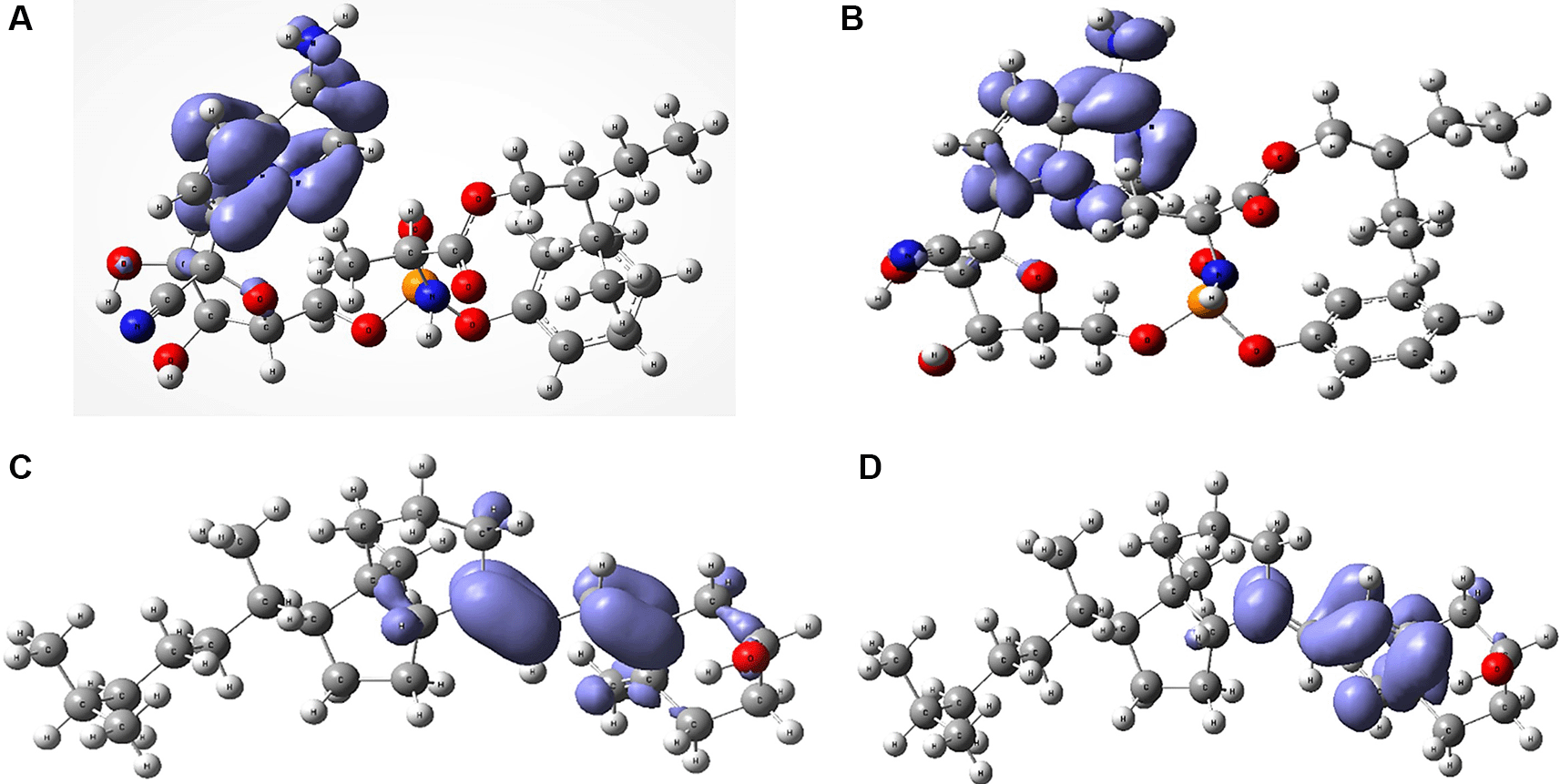
A. Fukui function and B. Fukui Function related with remdesivir. C. Fukui Function and D. related with cholecalciferol vitamin D.
Figure 6 and Figure 7 show the Fukui functions and for the compounds selected. The Fukui function is associated with the susceptibility of a site to being attacked by electrophilic species, while represents the susceptibility of a site to being attacked by nucleophilic species. In these figures, we can see the functions of Fukui (-) and (+) on the identical zones in the molecular structure. These can be related to the stabilization process on the active site, showing the zones associated with global reactivity indices like electrophilicity and softness. These reactivity parameters can help understand the destabilization process and the bond formation (-H bonds) in non-covalent interactions. All these outcomes can be useful for the rapid assessment of the currently available antiviral drugs used for treating COVID-19 patients, which is crucial at this time of crisis, as well as for discovering newer drugs.
In this study, a number of ligands related to the treatment of COVID-19 and used as inhibitors of SARS-CoV-2 virus, such as abacavir, acyclovir, amprenavir, ascorbic acid vitamin C, azithromycin, baloxavir, boceprevir, cholecalciferol vitamin D, cidofovir, edoxudine, emtricitabine, hydroxychloroquine and remdesivir, have been analyzed by molecular docking, molecular quantum similarity and chemical reactivity indices to study their active site stabilization interactions from a structural and electronic point of view.
In this study, to develop the docking experiments, some compounds were selected as references, these compounds are frequently related with the treatment of COVID-19. Some important anti-polymerase drugs were tested that are currently in clinical trials or on the market to stop viral infection on an emergency basis. The docking interaction for remdesivir, cholecalciferol vitamin D, azithromycin and ascorbic acid have shown good interaction (-H bonds) in the active site. The main idea is to extrapolate these outcomes and find novel insights into inhibitors for COVID-19.
The analysis of molecular quantum similarity indices on inhibitors showed high differences from a structural point of view. However, they are quite similar in their electronic density, obtaining the highest values in the electronic similarity index. Global and local chemical reactivity indices were calculated. These indices allow for the identification of the zones of chemical reactivity involved in the non-covalent stabilization of these inhibitors on the active site. Moreover, new outcomes about compounds such as abacavir, which is used to treat HIV infection, were shown and some studies have shown their relationship with COVID-19. In addition, it sheds light on the use of novel ligands for the treatment of this challenging disease that has claimed millions of lives worldwide.
Protein Data Bank: SARS-Cov-2 RNA-dependent RNA polymerase in complex with cofactors. Accession number 6M71; https://identifiers.org/pdb:6M71
Harvard Dataverse: New insights in series of ligands used as SARS-CoV-2 virus inhibitors within molecular docking, molecular quantum similarity, and chemical reactivity indices frameworks. https://doi.org/10.7910/DVN/MSIGUS.51
Data are available under the terms of the Creative Commons Zero D. “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular Biology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Phytochemistry, medicinal chemistry, and in silico research
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: DFT, MD
Is the work clearly and accurately presented and does it cite the current literature?
No
Is the study design appropriate and is the work technically sound?
No
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Bioinformatics, Immunology, Data Sciences, Computational Biology
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Computational chemistry; DFT; Nanotechnology; Docking simulation
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Computational chemistry; DFT; Nanotechnology; Docking simulation
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Computational chemistry; DFT; Nanotechnology; Docking simulation
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
|
Version 3 (revision) 11 Dec 23 |
read | read | read | read | read |
|
Version 2 (revision) 10 Aug 23 |
read | ||||
|
Version 1 09 Aug 22 |
read | ||||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)