Keywords
Intellectual property rights, Patent landscape, Stem-cell therapy
This article is included in the Cell & Molecular Biology gateway.
Stem cell technology is a powerful tool ready to respond to the needs of modern medicine that is experiencing rapid technological development. Given its potential in therapeutic applications, intellectual property rights (IPR) as a protection resource of knowledge are a relevant topic. Patent eligibility of stem cells has been controversial as restrictions to access the fundamental technologies open a gap between research and clinic. Therefore, we depicted the current patent landscape in the field to discuss if this approach moves forward in closing this breach by examining patent activity over the last decade from a transdisciplinary perspective. Stem cell therapeutic applications is an area of continuous growth where patent filing through the PCT is the preferred strategy. Patenting activity is concentrated in the USA, European Union, and Australia; this accumulation in a few key players leads to governance, regulation, and inequality concerns. To boost wealthiness and welfare in society - stem cell therapies' ultimate goal - while at post-pandemic recovery, critical elements in the field of IPR rise to overcome current limitations: to promote bridge builders able to connect the research and business worlds, regulatory updates, novel financing models, new vehicles (startups, spinouts, and spin-offs), and alternative figures of intellectual property.
Intellectual property rights, Patent landscape, Stem-cell therapy
Attending the reviewer’s observations, we included comprehensive information and discussion on relevant issues:
1. Provide further reasoning and evidence around the role and impact of patenting in stem cell therapy and a nuanced discussion on the potential risks and drawbacks of alternative IPR.
2. Included references for a deeper understanding of technological patent trends around iPSC technology a decade after their creation (2006-2015).
3. We updated data on Figures 1, 2, 3, and 5 to include the up-to-date information for patent documents filed in 2020.
See the authors' detailed response to the review by Pratap Devarapalli
See the authors' detailed response to the review by Verónica A. Palma
Innovative scientific discoveries, such as cell therapies, are routinely used among clinicians in their medical practice.1 Thus, cell therapies are powerful tools ready to respond to the needs of modern medicine. Furthermore, stem cell therapies face the challenge of improving the quality of life in rapidly aging societies.2 Due to the rapid technological development cell therapies are experiencing and their high potential in therapeutic applications, intellectual property rights (IPR) as a protection resource of knowledge is a relevant topic.
Stem cells are traditionally defined as undifferentiated cells with unlimited potential to regenerate cells or tissues lost due to disease and thus restore normal function.2 However, more recent studies have shown stem cells to have reparative properties, homing to injury sites and stimulating tissue repair.3
The clinical relevance of stem cells has resulted in the development of clinical trials evaluating the adequacy of different lineages of stem cells for a variety of specific therapeutical applications such as hematopoietic stem cell transplantation for blood disorders,4 mesenchymal stem cell therapy for Graft vs Host disease,5 retinal pigment epithelium derived from iPSCs for age-related macular degeneration.6 Some of them are being approved for commercialization under the US7 and European Union jurisdictions.8 Those remarkable discoveries beg the question of how they can be protected and to what extent by IPR.9
The patent eligibility of stem cells – particularly those derived from human embryos and human embryonic stem cells (hESC) – has long been debated in scientific and legal communities. However, precedents established in USA courts significantly narrow the scope of patent eligibility within biotechnology. The implications of recent legal changes on stem cell patent eligibility have already been compared in the European Union (EU) against those applicable to the USA.10
Current research has analyzed the challenges for patents based on human stem cells with therapeutic uses and patentability limits.11,12 In addition, the comprehension of state of the art has been analyzed from the legal perspective of applicable regulations in different regions and jurisdictions (Europe, the USA, China, and Japan), considering ethical issues and relevant regulatory restrictions.13
International courts have widely treated cases and controversies around patent activities in stem cell technology. An emblematic case is the controversies and legal disputes derived from a family of three patents held by the Wisconsin Alumni Research Foundation (WARF) that covered the first isolation of nonhuman primate stem cells and hESC. The court considered the claims “overly broad and restrictive inhibiting researchers' access to stem cell lines due to high licensing costs”,14 falling within the “Alienation Phenomena of Biotechnology Patents,” where excessive patenting restricts researcher access to the essential technologies to go further.15
While surfing the intellectual property outlook, researchers face legal uncertainties, high costs, and limitations on data sharing.16 Even a diligent stem cells researcher or entity that wishes to respect IPR will face uncertainty and enormous expenses in dealing with the IPR landscape.16,17 Furthermore, it is antithetical for one institution or company to hold a “universal patent” that, when licensed, provides total freedom to operate,18 limiting the forthcoming of the promising industry of stem cells.
WARF's patents show how despite limitations, the patent system works in conjunction with robust, nonprofit, and primarily publicly funded scientific research institutions.19 However, the field of stem cell therapies is not a bidirectional relationship between academia and private companies. Instead, it is a complex ecosystem influenced by government policies and court rulings in their respective jurisdictions,14 assembled over a translational model where researchers from the benchside, health professionals from the bedside, communities of healthy populations, and patient groups work together20 to boost wealthiness and welfare in society.
Controversy aside, from looking at this outlook, one question arises: is the current arrangement of the patent system a pathway for closing the gap between research and clinic? Therefore, we reviewed the patent landscape of stem cells, the primary tool of various cell therapies, over the last decade (2011-2020) to understand how the patenting activity behaved by analyzing trends in patent records and the stakeholders' contributions. On these grounds, we integrate a transdisciplinary perspective that allows researchers, decision-makers, and investors, to have a broad panorama to effectively address essential factors to enable equal access to technology, tackle the governance challenges, and provide IPR alternative strategies.
As patent data represents inventive activity, in this work, we explore innovations in stem cell therapy over the last decade by analyzing the patenting activity reported at The Lens Patents and PatentScope, the WIPO's repository.21 PatentScope is an official source of information that encompasses several patent authorities with sufficient technical resources to explore state-of-the-art technology in a particular field.22 The Lens Patents is a collaboration between the non-profit Cambia and Queensland University of Technology that offers one of the largest patent literature and citation indexes available.23
Landmark cases have shaped the patent frontiers in different jurisdictions: while WARF vs Consumer Watchdog case in the US upheld the patentability of human embryonic stem cells, in Europe, Brüstle vs Greenpeace case ruled out the patentability of hESC. Both WARF’s and Brüstle’s patents controversies are the clearest examples of how the scope of IPR and policy contexts affect stem cell technology.
WARF is a nonprofit foundation that manages intellectual property generated by researchers at the University of Wisconsin at Madison. In the mid-1990s, James Thomson and coworkers developed an approach to maintain long-lasting ESC lines from two species of primates. Afterward, in 1998, the group created an analogous hESC; due to governmental prohibits for using federal funds in research with human embryos, Geron Corporation funded the research in exchange for exclusive and nonexclusive rights under patents that might result. The first application for the IPR patent family was filed by WARF in the US in 1996 and awarded in 2006. However, in 2004 the European Patent Office (EPO) refused the application on moral grounds. While in the US, the controversy around WARF's patent family was centered on technical issues alleging obviousness and lack of novelty, in Europe, WARF's efforts to protect their inventions through the European Patent Office (EPO) failed due to morality concerns as the only means to obtain hESC with the claimed method involved destroying human embryos, making the method unpatentable under the Rule 28 of the European Patent Convention.19
WARF's protection strategy unleashed aggressive critics and accusations of asserting control over a primary science platform needed for health-related research. WARF was challenged by Consumer Watchdog, a California-based consumer rights organization, on the grounds of obviousness at the USPTO in 2006 before the patent was granted. However, it was dismissed. In 2007 WARF liberalized its patents' licensing by eliminating the prohibition against academic researchers from sharing WARF's hESC and extending exemption on licensing fees; by 2009, they had completed 35 licensing agreements for hESC with 27 companies.19 In 2014, after the Leahy–Smith America Invents Act (AIA) was declared and one year before WARF’s patent expiration, Consumer Watchdog challenged USPTO's 2006 decision on the US Court of Appeals for the Federal Circuit appealing to the US Supreme Court's decision on Association for Molecular Pathology vs. Myriad Genetics Inc. on gene patenting. The US Court dismissed the petition again as there was no legal injury related to the patent, considering that Consumer Watchdog was never sued or threatened to be sued by WARF.24
The German scientist Oliver Brüstle filed an application for a patent entitled ‘Neural Precursor Cells, Method For The Production And Use Thereof In Neural Defect Therapy’ in 1997 in Germany. The patent, granted in 2006, intended to protect methods of converting hESCs into neural precursor cells to treat severe neural deficiencies such as multiple sclerosis, Parkinson’s, Huntington’s, and Alzheimer’s diseases. In 2011, Greenpeace made a case to annul the patent based on Article 6.2.c of European Biotech Directive 98/44/ec, where invention inventions using human embryos as base material are excluded from patentability.25
The Court of Justice of the European Union pronounced the sentence of the case in October 2011, resolving that “a process which involves removal of a stem cell from a human embryo at the blastocyst stage, entailing the destruction of that embryo, cannot be patented”.26 This decision excludes from patentability the uses of human embryos for industrial or commercial purposes, impeding the market entry of hESC-based products through this pipeline,27 but opens the door to patents on embryonic stem cell products that did not involve the destruction of an embryo28 and to take advantage of other forms of IPR protection to break barriers and allow commercialization of these therapeutical alternatives.25
Despite the challenges, inventive activity around clinical applications of stem cells has been growing during the last three decades, reflected in the steady growth of patent filing activity in stem cells since the 1990s.29 A peak and drop in the first decade of the 21st century were observed, being at a stable pace until 2010.16 However, by 2010, the hype of stem cell technology had dropped: added to the ethical and sociopolitical controversy, the timespan for these technologies to reach the clinic, the slow entrance of industry into the field, and the lack of business models specifically engineered for stem cell-based therapies, and regulatory uncertainties created a hard shell for investment.16,18
The evolution in the number of patent documents (applications and grants) from 2011 to 2020 is presented in Figure 1. From 2011 to 2015, the number of patent documents produced annually remained relatively constant. However, as of 2015, a gradual increase in annual applications and grants began, presenting a maximum peak in 2021.
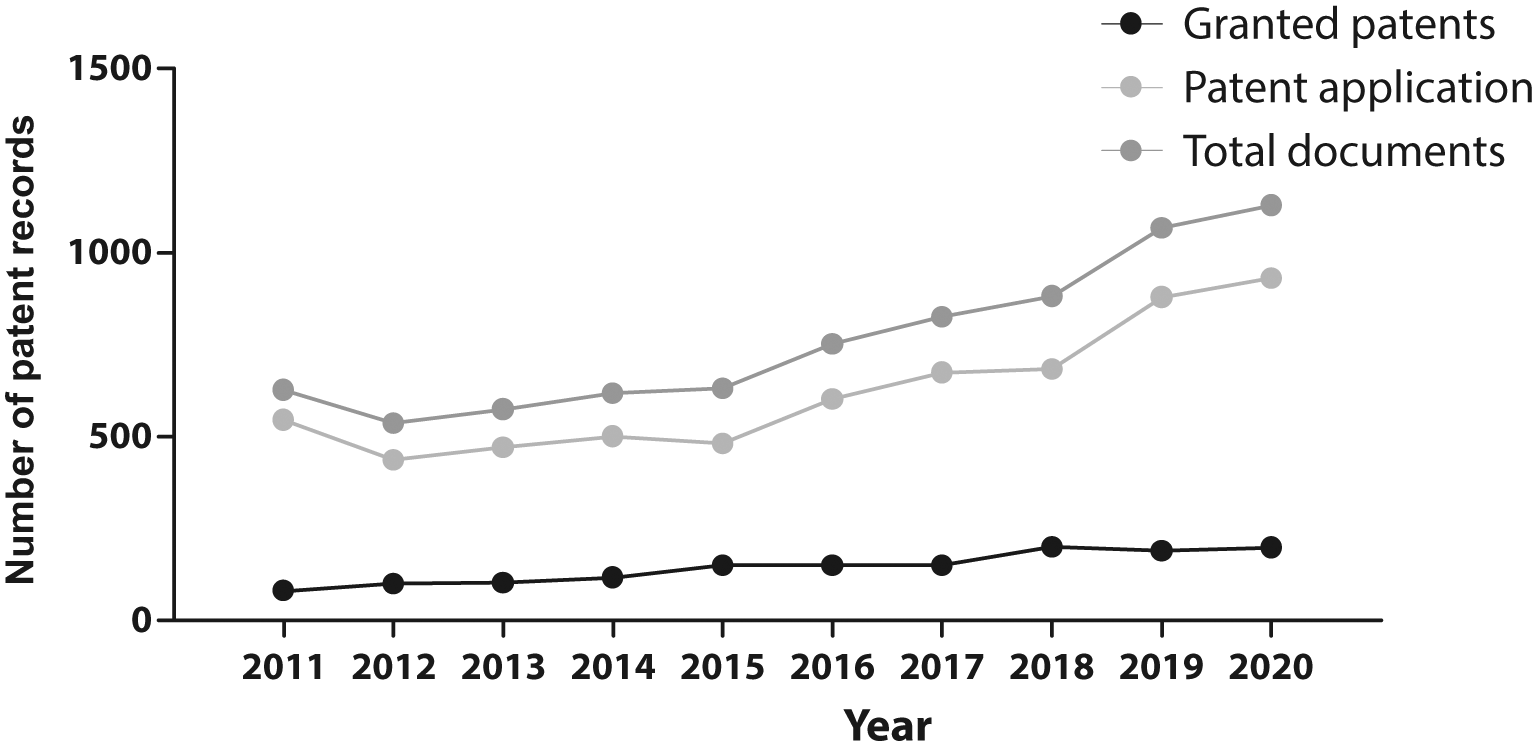
Own elaboration with data of The Lens Patents.30
In our research, the increase in the number of patent filings in the analyzed field in the last decade shows that this research field has been an arena of continuous growth and innovation, particularly since 2015. This switch resulted logically as discourses and policies changed during the first years of the decade. For example, in 2012, the Nobel Prize in Physiology or Medicine was granted to John B. Gurdon and Shinya Yamanaka for the discovery of reprogramming: mature cells can be reprogrammed to become pluripotent,31 enforcing this new avenue in stem cell therapy; also, in the US, during Obama's administration the AIA was enacted, changing the game in stem cell patent activity, making it a more attractive and competitive environment as it makes more accessible and cheaper to challenge stem cell patents as they become issued.24 After that, in 2014, the EU overturned laws that banned stem cell patents, allowing patent granting if the biological materials are accurately described and have an industrial application.9
Although the Nobel Prize was awarded in 2012, Yamanaka's research has been extended to iPS cells for new medical treatments since 2007. Research activities were based at Kyoto University in Japan's Center for iPS Cell Research and Application (CiRA). Success in generating iPS cells from human somatic cells was a breakthrough, and it overcame the ethical concerns surrounding the use of human embryonic stem cells for medical research because it made it possible to access pluripotent cells without destroying embryos. It also created many opportunities for medical research, particularly in diagnostics, drug screening, and regenerative medicine. Therefore, it is not surprising that in the period we analyze, an increase in patenting interest at the global level has been observed in the SC field. CiRA has pursued an IPR strategy to influence how the research results are used. Where appropriate, CiRA searched to secure patents over crucial technologies; its goal was not to monopolize iPS cell technology but to ensure that it is widely available for development by other researchers through reasonable, non-exclusive patent licensing arrangements. This way, they could control and stop others from holding it by patenting iPS cell technology. We emphasize the transdisciplinary nature of medical research, where many researchers tackle complex problems from various angles. CiRA believes it is essential that all researchers have access to iPS cell technology because of the possible discoveries that their research may lead to. A strictly exclusive approach that narrows the research and development base would likely translate into many lost opportunities for science.32
According to CiRA, high license fees risk constraining the advancement of iPS cell research and its availability for patient care. Research into iPS cells has attracted much attention and generated intense competition within the biotechnology sector. In addition, there is no guarantee that others will not seek to create a “patent wall” and lock the door on the technology. Therefore, by keeping the door open through its non-exclusive patent licensing approach, KU is trying to prevent this wall from being built.
From this experience on iPS cells, strategic management of IPR could ensure that they were widely available at reasonable and appropriate licensing fees and that iPS cell research is broadened and accelerated so that new drugs and treatment methods will be available to patients expeditiously.32
As in the 2000s, the inevitable sought for new technological platforms to reach the market has caused recent controversies around which landmark cases will shape the future of the patent arena in the field.
On one side, the decade-long dispute between Broad Institute (Feng Zhang, US) and the “CVC” (Jennifer Doudna and Emmanuelle Charpentier from the University of California, US and the University of Vienna, Austria) about who possesses exclusive patent rights for the foundational CRISPR–Cas9 genome-editing technology in eukaryotic cells (reviewed by Aquino-Jarquin33) has led to a dramatic increase in the patent applications claiming the use of alternative enzymes, smaller and easier to transport into human cells, such as Cas12 or Cas13,34 however, this IPR battle could potentially jeopardize CRISPR/Cas9 use in human therapeutical applications.35
On the other hand, the harmonizing agreements between iPSC pioneers are intended to allow iPSC platform to reach the market and improve human healthcare. iPSC was first created in 2006 by Shinya Yamanaka (Kyoto University, Japan) by reprogramming mice adult cells to an embryonic-stem-cell-like state using four genetic factors,36 achieving the same for human cells in 200737 and publishing his research the same day as James Thomson (University of Wisconsin-Madison, US).38 Cellular Dynamics International, Inc., a leading developer and marketer of next-generation stem cell technologies for drug development and personalized medicine applications, founded by Thomson in 2004, secured access to Yamanaka’s work39 via the iPS Academia Japan, Inc., an affiliate of Kyoto University responsible for managing IPR held by Kyoto University in the field of iPSC cell technologies. Since 2010, Cellular Dynamics International Inc. became the first company worldwide accredited to access the key patents surrounding iPSC technology through a nonexclusive licensing agreement for the fundamental Yamanaka’s iPSC patent portfolio.40
A look into the patent portfolios of the top three patent assignees in IPSC technology led by Devarapalli et al. (2016).41 evidenced patent trends and multiple factors that reflected the competitive scenario between the top assignees of IPSC technology, suggested that Kyoto University, led by inventor Shinya Yamanaka was found to be the leader of iPSC technology. However, patent-product linkage analysis suggested CDI, led by inventor James Thomson, may surpass Kyoto University quickly.
According to the findings of Devarapalli et al. (2016),41 until 2016, Yamanaka, the principal inventor of KU, started commercializing iPSCs technology by licensing out patents to more than 150 entities worldwide. As James Thomson (University of California) had turned into an entrepreneur from inventor and established CDI, the technology trends of UC have fallen with the subsequent rise of CDI in iPSCs. KU’s patent portfolio was comparatively strong, with most patents and an average citation per patent (ACP) value of 2.69 in significant breakthrough technologies of iPSCs research.
It was estimated that CDI would surpass KU, and having a license for using platforms for genomic editing CRISPR, ZNF, and TALEN from SC, in theory, could expand the applications and coverage in diagnosing and treating diseases. However, disputes, controversies, and interfering judgments on IPRs could hinder or slow the delivery of solutions to patients.
We integrated a core collection of published patent documents constructed to encompass all stem cell applications from the USPTO, the EPO, WIPO's Patent Cooperation Treaty (PCT) filing system (Figure 2). As in early 2000’s decade42 USPTO’s filings represent the majority of global patent documents, followed by the PCT domination. The fact that patent documents are being filed through the PCT suggests the intention to protect the invention simultaneously in different countries.
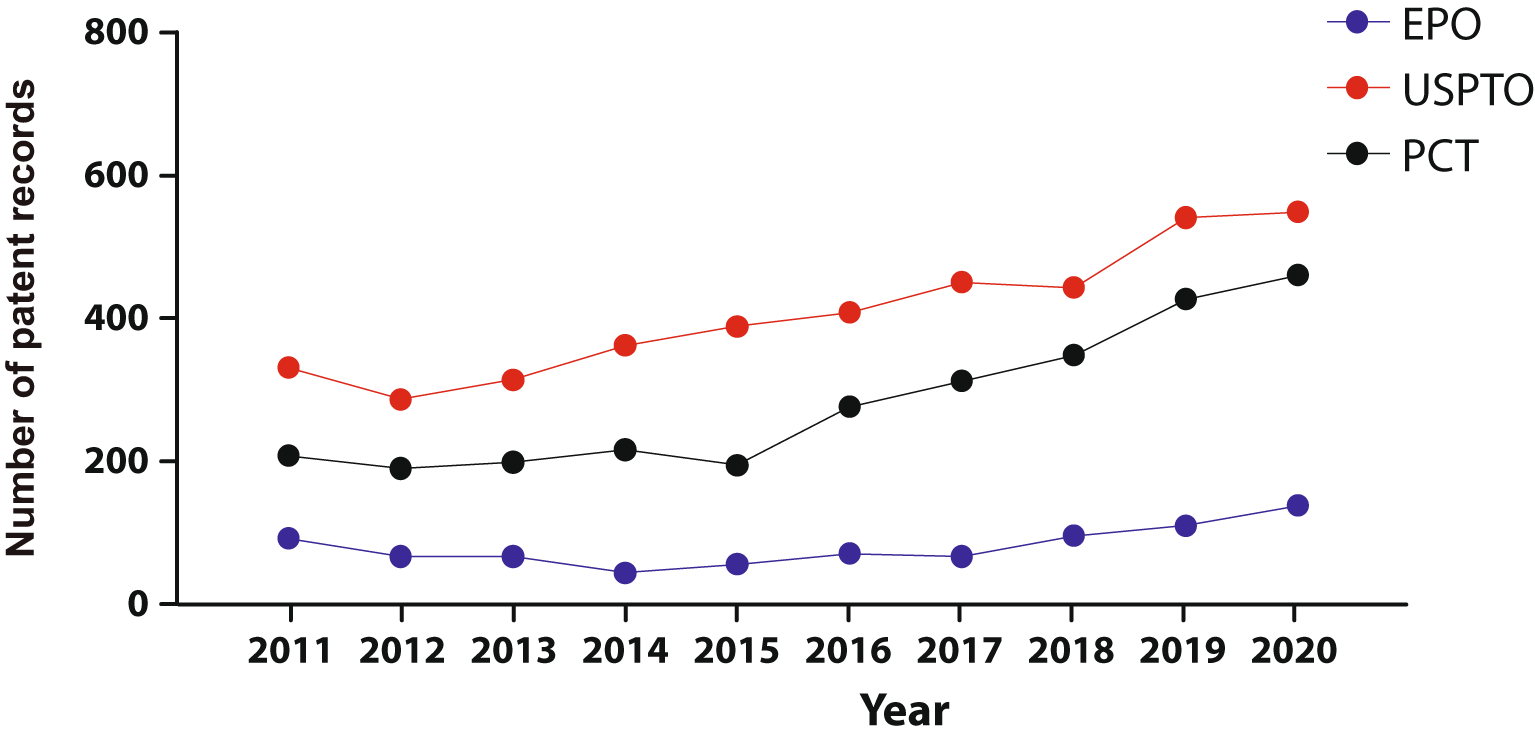
Own elaboration with data of The Lens Patents.30
When stratified by filing national offices (Figure 3), the United States of America remain as the most prominent target market, followed by the EPO. Hungary, United Kingdom, Israel, Canada, and China place as the leading markets in the patent arena. Other jurisdictions with less than 10 patent documents in the studied time frame include Asian (Republic of Korea, Japan), European, (Turkey, Denmark, Finland, Sweden, Ireland, Belgium, Estonia, Poland, Portugal), and Latin American countries, as well as Egypt, New Zealand, and the Russian Federation.
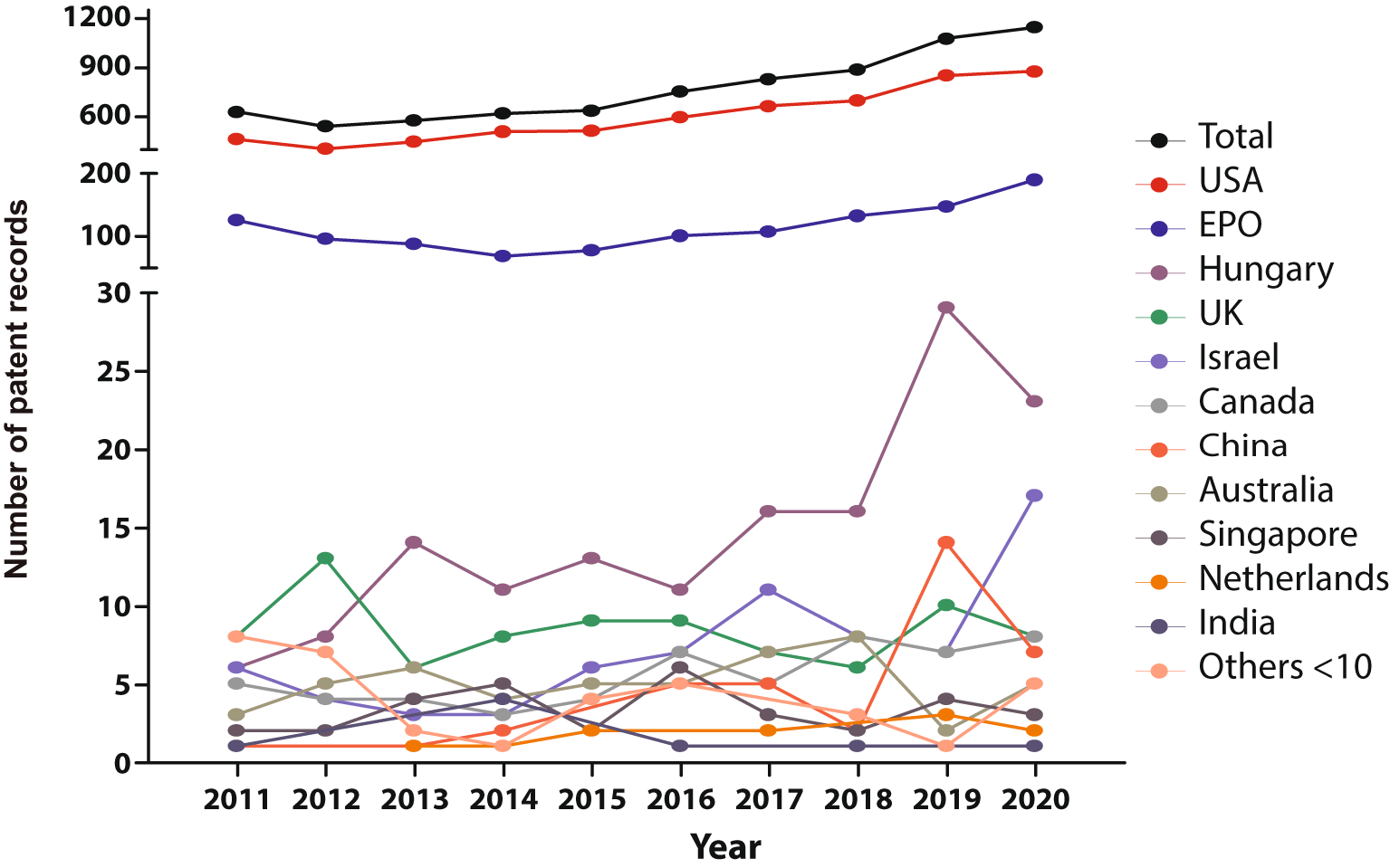
Own elaboration with data of The Lens Patents.30
CiRA as a leading research centre in advanced therapies on stem cells believes that all iPSC technology should be available to all people, regardless of nationality. For this reason, they have been promoting to obtain patents in as many countries as possible through the PCT. As of May 2015, Kyoto University held patents relating to iPSC technologies in 30 different countries. Like other universities and research institutes with limited financial and human resources, Kyoto University has taken advantage of the PCT’s simplified and cost-effective procedures. Using the PCT also provide CiRA more time to assess whether we really need to patent a given technology. Despite its many advantages, however, one point of frustration in using the PCT is the fact that it is not possible to receive identical patent rights in each of the countries in which we seek patent protection. This constraint is related to the significant variations between national patent laws and examination practices.32
The United Kingdom National Stem Cell Network (UK NSC) patent watch landscape is a dataset of published patent applications and granted patents to provide a bigger picture regarding the patenting of stem cell technology. However, the UK NSC patent watches dataset is limited to published applications with WO, US, EP, and GB designations, and the granted patents on the USA, EP, and GB. Hence, to place the results of the UK patent watching a more global context and to give a fuller picture of the worldwide activity concerning stem cells, an overview of the complete global dataset would be beneficial given the recent rise in worldwide patent filings from countries such as China and India.43
It is worth to mention that both USA and European Union have made changes throughout the last years to include stem cell-based and other advanced therapies within their regulatory framework, which not only contribute to understand the rise in patenting activities after 2015. The US 21st Century Cures Act (2016) considers stem cell product commercialization under the Regenerative Medicine Advanced Therapy (RMAT) designation. RMAT designation allows FDA accelerated approval based on a surrogate endpoint, such as clinical benefit. A drug is eligible for RMAT if three conditions are fulfilled (1) it is a regenerative medicine therapy (cell therapy, therapeutic tissue engineering product, human cell and tissue product, or any combination product), (2) “intended to treat, modify, reverse, or cure a serious or life-threatening disease or condition”, and (3) potential to address unmet medical needs is demonstrated by preliminary clinical evidence.44 Minimally manipulated cellular products are exempt from this regulation under the Code of Federal Regulation (CFR) Title 21 1271.10(a)(1).45 On the other hand, in the European Union stem cell therapeutical products are encompassed within the Advanced Therapy Medicinal Products (ATMP) classification (2007) under the Priority Medicines (PRIME) designation (2016). PRIME designation is granted by EMA to medicines that target public health unmet medical needs by offering “early and proactive support to medicine developers to optimize the generation of robust data on a medicine’s benefits and risks and enable accelerated assessment of medicines applications”.46
Japan has been a substantial actor in the stem cell patent during the last decades.24,30,47 Even if not listed in our research among the top 5 most active, Japan's policies have fostered stem cell innovation. Liberal Democratic Party, elected in 2012, strongly supported research in the field with over 220 million US dollars as part of a stimulus package to lift the Japanese economy from recession.48 In Japan’s regulatory framework stem cell therapies are considered within three instances: the Sakigake designation, the Act on the Safety of Regenerative Medicine (RM Act), and the Pharmaceuticals and Medical Devices Act (PMD Act).49,50 First, Sakigake designation (2015) is granted by Japan’s Ministry of Health, Labour and Welfare (MHLW) through the Pharmaceuticals and Medical Devices Agency (PMDA) for innovative products with intended use for a serious disease with significant impact on life and prominent effectiveness, planned to be submitted of premarket application firstly in Japan, with consideration of its development in Japan from an early stage.49 Second, the RM Act encompass the use of processed cells for clinical research and medical practice guiding its administration to patients, comparable to US CFR Title 21, and considers a licensing scheme to stablish cell processing facilities outside health institutions.50 Last, the PMD Act provides a specific regulatory framework to commercialize regenerative medicine products by granting time-limited marketing approval demonstrated probable benefit and proven safety.50
Altogether, stem cell regulatory frameworks in US, EU, and Japan incentives innovation by favoring the commercialization of cell-based alternative therapeutical approaches trough the development of mechanisms to accelerate the bench to bed translational process. This strategy is intended to allow patients to reach these therapies earlier and significantly improve their quality of life while protecting them from harmful unapproved or unproven interventions.51,52 Nevertheless, the rapidly changing in regulatory pathways raise new questions in scientific, ethical, and medical fields that are still under debate.53 Through time, these storylines change the relevance of policy designs, reinforcing the notion that patent law, national policies (Economic, Educational, and Science-Technology-and-Innovation) affect how novel technologies are fostered to attain wellbeing in society.
Table 1 presents the institutions and private corporations with more patent productivity on stem cell therapies - with more than 80 contributions each. In this top ten, seven applicants belong to academia, and four can be classified as private corporations. Four institutions are highly prestigious USA universities within academia, and two are research centers (Memorial Sloan-Kettering Cancer Center, Fred Hutchinson Cancer Center). In addition, three organizations are large pharmaceutical companies (Fred Hutchinson Cancer Center). In addition, three organizations are large pharmaceutical companies (Immunomedics Inc., Regeneron Pharmaceuticals, and Novartis Ag) and a research-focused hospital (The General Hospital Corporation DBA Massachusetts General Hospital).
Own elaboration with data of The Lens Patents.30
Interestingly, the largest biomedical companies do not appear within the top the patent applicants, however, when we look to the bigger picture, four of the major players in the pharmaceutical industry are found within the top 50: Janssen Pharmaceutical (no. 20, 29 documents), Bristol Myers Squibb (no. 22, 27 documents), Roche (no. 19, 29 documents), and Pfizer (no. 50, 15 documents). Bayer, GlaxoSmithKline, Sanofi, Abbott Laboratories, and AstraZeneca also are listed with less than 10 documents each. All of the above-mentioned companies appear as patent owners or withing the licensing records for the period.
Figure 4 illustrates how patent documents are distributed among holders. Academia-related institutions hold 33% of the patent documents. The dominant presence of universities in the field suggests that, as stated above, long incubation periods within academia are required for this technology to be ready. These results represent a shift in trends from 2000’s when 56% of the patent ownership has concentrated in private institutions in which only 8% was represented by inventors, and the remaining 44% was held by public governmental institutions and academia.24
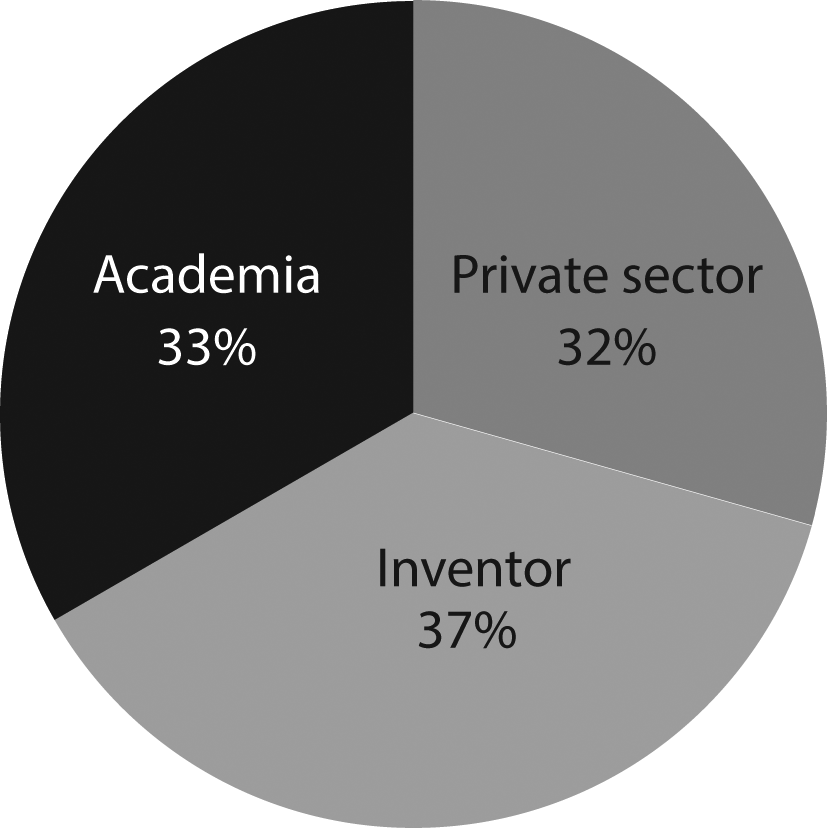
Own elaboration with data of The Lens Patents.30
From this, we may sketch the three key players that have emerged in the field. First, academia, including Universities and Research Institutes, plays a crucial role by generating and disseminating knowledge around stem cell research. Through fundamental research, often funded by government agencies, academia seeks to understand the biology and potential applications of stem cells. Traditionally, universities and research institutes protect their researcher’s inventions through patents to commercialize or license the finding to private companies for further development. Second, government agencies in different jurisdictions have played a significant role in funding stem cell research and promoting innovation in the field guided by policy agenda around regulations for stem cell research to promote ethical practices and responsible use of stem cell technologies. And third, private companies have actively engaged in stem cell research and development, focusing on translating scientific discoveries into commercially viable therapies and technologies, by filing or securing IPR, which grants them a competitive advantage in the market. The patent portfolios of private companies can significantly impact the landscape of stem cell research and influence the direction of innovation.
From 2011 to 2015 the majority of applicants were individuals, however, since 2015 a shift trend is reported when academic institutions and private companies lead the field of patenting activities (Figure 5). While public institutions often prioritize openly sharing knowledge to build research upon existing discoveries and advancements, the increment in patent documents filed through the last decade shows how academic institutions are engaging in technology transfer activities. Therefore, technology transfer offices and licensing agreements could be fundamental in bringing stem cell technology to society.
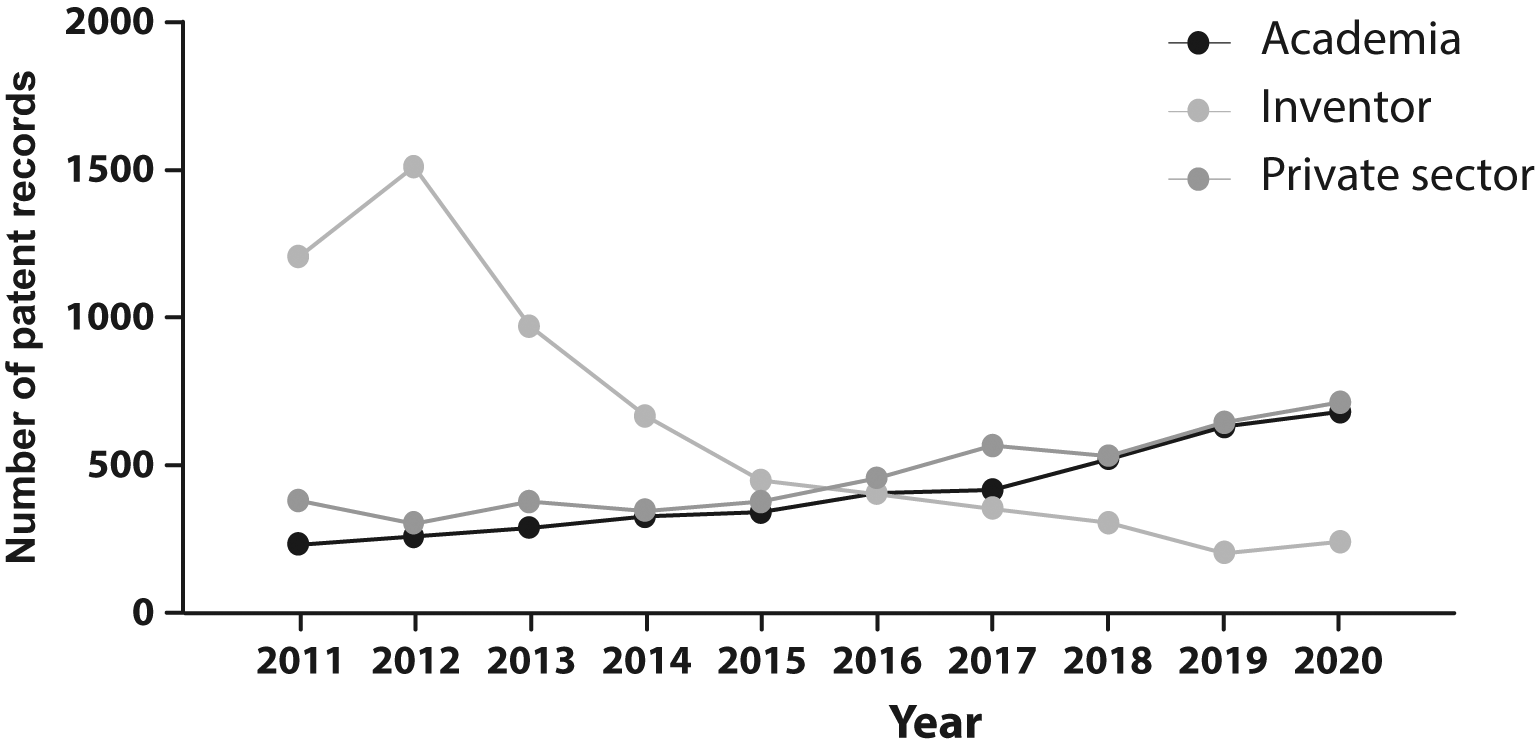
Own elaboration with data of The Lens Patents.30
A recent patent analytics report of the Centre for Stem Cell Systems of the University of Melbourne investigated technology development related to mammalian pluripotent stem cells.47 Patent families in this technology are predominately directed at differentiation (28%) and stem cell production (26 %). The high number of patent families granted or with protection being sought (98%) for stimulation and tissue engineering indicates the importance of patent protection in this technology. This analysis showed that innovation in mammalian pluripotent stem cells is dominated by universities and research institutes and has a healthy level of collaboration, which indicates that the technology is in the early stages of development. Nevertheless, it is a growing field with enormous opportunities for translating research into applications as technology matures.
A clear strategy to achieve this migration from bench to bedside can be depicted from analyzing the IPC codes under which inventions are indexed. Figure 6 illustrates the 10 more frequent codes for stem cell patents in the last decade. IPC A61K is assigned to inventions classified as compositions for dentistry, cosmetic, or medical purposes. IPC code C12N encompasses microorganisms, enzymes, and compositions that include substances produced by or derived from, microorganisms or animal cells. A61P IPC classification covers the therapeutic activity of chemical compounds or medicinal preparations already classified as such in subclasses A61K or C12N. Altogether, this classification strategy suggests that potentially commercialized products are assembled medicinal preparations of stem cells, or its derivates.
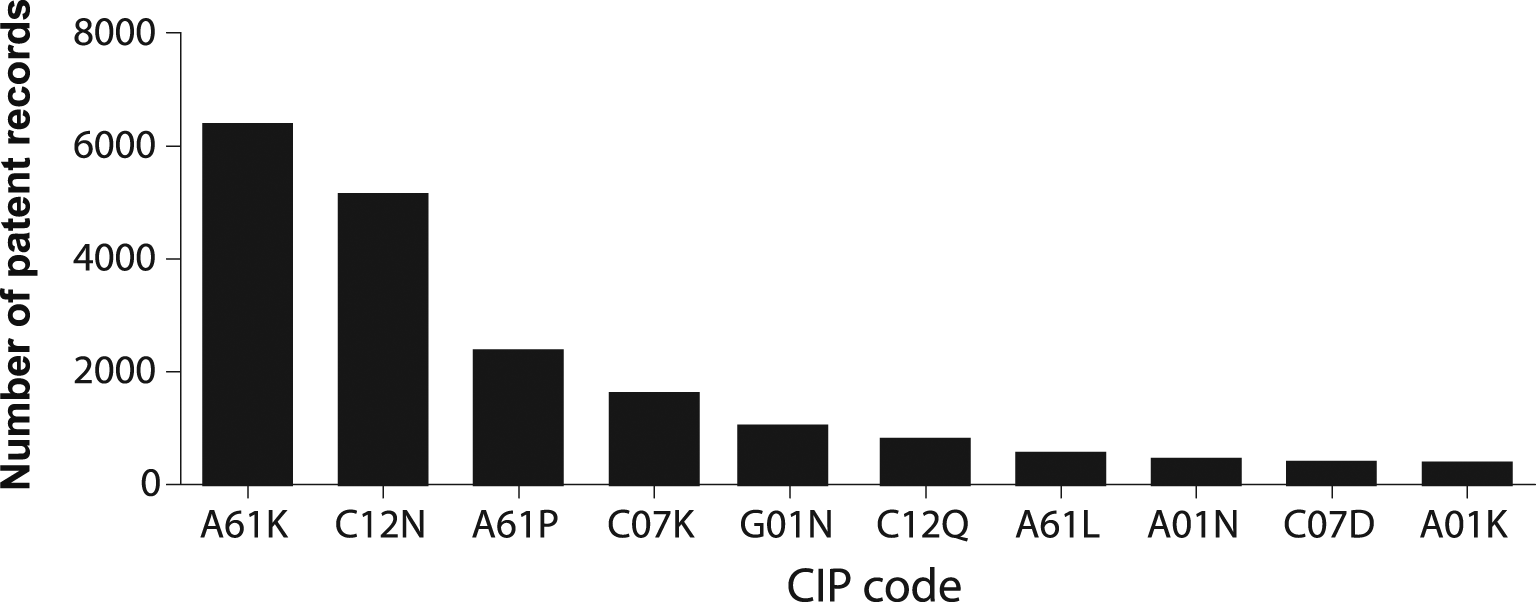
Own elaboration with data of The Lens Patents.30
Patent systems' primary purpose is to encourage innovation to achieve social welfare by granting exclusive rights over an invention if this fulfills three criteria: novelty, utility, and non-obviousness. Hence, the nature of a patent is to generate profits and gain control over the market, risking its original justification: social benefit.15
Our research shows that innovation in stem cell technology is concentrated within a few hands of the wealthiest sectors: (1) the eight nations with more patent filing activity are listed as high-level income countries by The World Bank,13 (2) nine of the most active organizations in the patenting filed are located at the USA, and (3) four of this top ten are private companies, whose logical-financial-interest for protecting its inventions is to make to most of it. So, how to balance access to stem cell technologies? As the global innovation landscape changes rapidly, a noticeable reshaping of the consumption and use of patent information happens. Brand new technologies, changing business needs, and evolving talent markets continuously affect the patent data's nature, shape, and transformative value. Here we discuss some keystones to consider for bridging the gap.
Stem cell and genomic research share controversies around IPR and policy contexts. However, they also have in common the complex and lengthy translational pathways of a complex innovation ecosystem based on working with human materials.16 After that, an essential lesson from genomics can be learned: the progress and use of technology improve its speed and quality while reducing associated costs.
In 2002, after an international 13-year effort, the Human Genome Project satisfactorily concluded, making publicly available 99% of 3000 million bases comprising the whole sequence of human DNA.54 After this breakthrough event, genome sequencing costs decreased by 10,000 times, from 1000 to 0.1 US dollars per megabase from 2007 to 2015.55
Like genomics before the Human Genome Project, stem cell research for therapeutical applications may be unattainable, so reducing the price of this technology becomes essential to impact the health and life of people around the world. A proposed strategy to achieve affordability for stem cell therapies are the stem cells biobanks.
Stem cell biobanks, public or privately funded,56 are highly specialized facilities aiming to collect and store human tissue samples to achieve isolation, storage, and long-term viability of human cells ex vivo.57,58 Biobanking can be beneficial to reduce costs, personalize therapies, and minimize patient inconvenience to access stem cell lines for both autologous and allogenic settings.59 Ideally, stem cell biobanks will be centralized entities with national and international legal counseling to work under harmonizing ethical protocols and unified international legislation to guaranty the purity and safety of the biorepository to assure the highest quality regarding the stem cell lines offered to interested researchers and clinicians.56,58,60
Since early 2010’s, USA, Japan, and European governments (Germany and UK) have been the primarily sponsors for biobanks.56 In USA, majority of biobanks are developed within academic and medical research institutions or tied to public health delivery systems, yet private funded smaller scale and disease-specific biobanks have also proliferated.61
Stem cells biobanks are designed to be scientific-driven entities that provide reliable and high-quality human stem cells to push forward innovative research and novel cell-based therapies by making cellular products readily available.57,58,62 However, early biobanking initiatives have been hinder by lack of governance frameworks, the influence of local experience for public trust, and concerns about financial sustainability.56
As he novel regulatory models for cell-based therapies are promising to revive interest in biobanking, probably funded by private institutions, these challenges need to be tackled by drawing clear paths around IPR and developing congruent business model.18,63 As proposed by Chalmer and colleges: “redefining commercialization activities and focusing on the creation of knowledge rather than commodities realigns commercialization with notions of public good”.56
A decade ago, the field of Regenerative Medicine – stem cell therapies included - was dominated by small biotechnology companies focused only on tools and nontherapeutic products or their services and manufacturing.16 However, our findings suggest a shift in this behavior, not only because the two medical facilities focused on clinical research rank among the leaders in the field, but, more patents have been filed under more than one category, and selected IPC codes relate to medical preparations and therapeutical activities of compounds or preparations, implying that on-the-edge technologies tend to incorporate different innovations to achieve a therapeutical alternative.
Next-generation engineering approaches for stem cell therapeutical applications includes gene therapy delivered to edit genome and epigenome stages, synthetic biology, and the inclusion of biomaterials for organoid generation and bioengineered tissues (reviewed by Bashor and colleagues).64
Advanced therapies involve a variety of inventions in many technical fields to manufacture a product, such as tissue selection, cell isolation, purification, culture, and specific therapeutical modifications – lineage differentiation, genetic changes, co-culture, and scaffold assembling-, GMP and quality assurance, transportations strategies, and strategies to transplant into the patient. These are just some of the scientific breakthroughs needed to be assembled through diverse technological developments – potentially patentable - to manufacture a stem cell therapy product. Thus, patent strategies used to protect small-molecule compounds are not likely to work well in the stem cell field; a technology portfolio is essential to cover and protect a commercial product.65
A possible strategy to integrate these patent portfolios and seize opportunities to expand new business areas is to take advantage of the existing technological strengths of different institutions. However, under the current patent systems, these results are unthinkable.
Despite CiRA achievements, iPSC is still far from ready for use in treating patients. There are still many hurdles to overcome in establishing reliable and safe treatment methods. These obstacles include peripheral technologies, such as cell quality evaluation and manufacturing methods, to ensure optimal iPSC development.32
Patents provide a legal framework that protects the rights of inventors, encouraging companies and researchers to invest time and resources into stem cell research. The promise of exclusivity can drive innovation and experimentation in the field. Also, the competitive nature of the biotechnology industry, driven by patents, can lead to rapid advancements. Companies compete to develop novel stem cell therapies, driving the field forward.
The possibility of commercializing stem cell therapies involves the realization of clinical trials, pass through regulatory approvals, for an eventual marketing of those therapies to patients. Patented technologies are more attractive to investors and venture capitalists because they offer a potential return on investment through market exclusivity. Companies holding valuable patents collaborate with actors within academia and industry, leading to interdisciplinary research.
Technology transfer is the span to close the gaps between academic research, industrial applications that allow commercialization of the result, and research's ultimate purpose: social welfare. While patents incentivize innovation, they also raise concerns about accessibility and affordability. Patented therapies can be expensive, limiting access for patients, especially in regions with limited healthcare resources.
Licensing agreements and generic competition might occur to increase access to stem cell therapies by reducing costs, patent disputes and legal challenges are not uncommon, especially in cutting-edge fields like stem cell therapy. Companies need to navigate these challenges to protect their innovations and market share. Typical licensing agreements had proved wrong for biotechnologies - as stem cells - to be transferred. The enormous fees involved hinder innovation instead of promoting novel developments – as WARF patent licensing fees slowed the advance of stem cells for a timespan.66 Hence, new ventures for technology transfer promise to close the gaps: academic spin-offs to transfer research to industry, corporate spinouts to share technology between private companies, and internal company start-ups to overcome innovation barriers within corporations.67 Nevertheless, endless jigsaws can be arranged based on those approaches.
In the early 2000s, financing stem cell research with strategies based on classic pharmaceutical models resulted in a “disappointing commercial history of cell therapy and contributed to the cool reception stem cell companies receive from venture capitalists”.18 However, the scenery changed after these new models matured: “some of the largest rounds of venture capital ever seen went into 2019 biotech startups”.68
New stem cell arose start-up companies, whose individual funding accounts for no less than 2 million US dollars, are scattered around the world, with 18 in North America (16 in the USA and two in Canada), seven in the European Union (three in the UK, one in Belgium, Netherlands, Germany, and Switzerland), three at Israel and only one in India.69 By analyzing the behavior in patenting activity of these stem cell-based start-ups, we can give a closer picture of the landscape in this emerging field. The scatter plot in Figure 7 presents patent portfolio size compared to funding amount for the top ten start-ups; funding data was retrieved from Medical Startups69 at the same time, patent information was collected from the Lens patent database30 by searching the number of patent records and families for each patent stakeholder listed (start-ups and associates). The trend shows more funding for start-ups with more solid patent portfolios – more patents with broader cover-, such as Celularity, Century Therapeutics, and Via Cyte; opposite to Rubius Therapeutics, a startup with limited patent families receiving more funding, and Cellular Dynamics, a less funded company with more patent families. Other start-ups listed within the top ten show emerging patent portfolios with more recent patent applications and families, ranging from 1 to 35 patent documents. However, optimistic as this may sound, future challenges will arise when these recently created enterprises consolidate as freedom-to-operate entities with existing patent grants.
Celularity, a $290 million funded Celgene Corporation spinout with a mature patent portfolio, aims to use placenta-derived stem cells as an alternative approach against blood cancer by developing therapies across autoimmune, degenerative disease, immuno-oncology, and functional regeneration. Noteworthy, Celgene Corporation performed as the seventh most productive institution in the stem cell patent filling landscape.69
Patents provide a legal framework that protects the rights of inventors, encouraging companies and researchers to invest time and resources into stem cell research. The promise of exclusivity can drive innovation and experimentation in the field. Also, the competitive nature of the biotechnology industry, driven by patents, can lead to rapid advancements. Companies compete to develop novel stem cell therapies, driving the field forward.
The possibility of commercializing stem cell therapies involves the realization of clinical trials, pass through regulatory approvals, for an eventual marketing of those therapies to patients. Patented technologies are more attractive to investors and venture capitalists because they offer a potential return on investment through market exclusivity. Companies holding valuable patents collaborate with actor within academia and industry, leading to interdisciplinary research.
While patents incentivize innovation, they also raise concerns about accessibility and affordability. Patented therapies can be expensive, limiting access for patients, especially in regions with limited healthcare resources.
While licensing agreements and generic competition might occur to increase access to stem cell therapies by reducing costs, patent disputes and legal challenges are not uncommon, especially in cutting-edge fields like stem cell therapy. Companies need to navigate these challenges to protect their innovations and market share.
Potential risks and drawbacks for these alternative strategies also need to be considered. First, IPRs over non-patented inventions, protected through brands, trade secrets, or other alternative models, may be subjected to legal disputes between the stakeholders considering the difficulties in legal recourse if a breach occurs, as employees or collaborators might inadvertently or intentionally disclose information, conferring IP a limited duration of protection. Second, by not subjecting inventions to authority, ethical dilemmas may arise around the development or testing processes of novel technological platforms. Third, proper financial models based on sustainable technical valuation and market-based validation will be required to manage the risks of market failures. Although these novel alternatives for IP protection and technology transference aim to allow advanced therapies to reach the market and boost social welfare, market-driven pricing may lead to social inequalities. Therefore, work must be done from different fronts to ensure these options are available to everyone.
As in any technological project, aligning research and development efforts with the goal of sustainable commercialization doesn't adhere to a straightforward trajectory and specific factors (as regulatory policy, federal funding, etc.) evolve over time, influencing the outcome of this technologies, positively or negatively, regardless the inherent market uncertainty of innovation. The need for timely decision-making within an environment of limited resources (economic, time, human, among others), as proposed by Cedano and Hernandez-Granados,70 will require tools to define the risk of technological projects at early stages of development through strategic information.
Undoubtedly, patents have been a stairway for stem cell research and development to access commercialization; however, the stairs for these products to evolve into therapies that enable social wellbeing are missing in this blueprint.
In certain jurisdictions, due to the lack of congruent regulatory frameworks to use IPR as a protection resource of knowledge, nondisclosure mechanisms of confidentiality and trade secrets are the only, but not preferred, options available to protect innovations in the stem cell field14; relegating patents as outdated mechanisms to bring stem cell technologies into the market. From this ground, we hypothesize: what if alternative figures of intellectual property for stem cell technology can overcome the current challenges?
Novel business models use stem cells as the cornerstone for cutting-edge technologies – 3D printing, bioprinting, organs on a chip, and genomic edition – that need a whole IPR-protected toolset to tackle real problems of rapid aging societies. As presented before, advanced therapies require a variety of inventions in many technical fields to manufacture a product, and patent portfolios are essential to cover and protect a commercialized product. Unfortunately, these systems may hamper innovation and development of stem cell technologies; thus, to overcome these limitations, the surge of protection strategies through trademarks, utility models, copyright, or creative commons are alternatives that close the gap.
Translating research outputs to economic and social benefits is highly challenging and requires a combination of expertise and bridge builders to connect the research and business worlds. Moreover, the challenge of commercializing or translating research into meaningful therapeutic applications has become even more critical as the global community needs to build momentum toward post-pandemic recovery. Therefore, those with expertise in the field must be proactive and work cohesively to improve their knowledge base. These challenges include constructing a more robust conceptual framework and improved metrics around knowledge transfer. A combination of qualitative research, vehicles that can bring that research to the market, startups, spin-offs, spinouts, SMEs, large enterprises, or other entities, and bridge-builders able to connect the worlds of research and business.71
By analyzing patent activity over the last decade (2011-2020), it becomes evident that limitations notwithstanding stem cells are an area of continuous growth and innovation, evolving in the assembly of technological portfolios to design therapeutic applications patented under diverse IPC codes. The USA, European Union, and Australia are attractive regions for inventive activity protection in the field considering the maturity of their patent systems, leading to patent concentration within a few critical stakeholders with broad coverage through the PCT pathway and concerns about governance regulation, future risks, and inequality. Critical elements are required to build bridges from research to market in a post-pandemic recovery where the global community needs to create momentum. Regulatory updates, novel financing models, new vehicles (start-ups, spinouts, and spin-offs), and alternative figures of intellectual property (nondisclosure mechanisms, trademarks, utility models, copyright, or creative commons) shape plausible avenues in the field of IPR achieve stem cell therapies' ultimate goal, boost wealthiness and welfare in society.
Patent data was obtained from Lens Patents or WIPO’s patent database Patentscope (https://patentscope.wipo.int/search/es/search.jsf) on March 2021 through the Advanced Search tool with the query: “CL: ((stem cell* NEAR10 (treat* OR transplant*)) ANDNOT ALL: (“plant” OR “vegetal”))”.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Patent law, Patent analytics, Genomics, Synthetic biology
Competing Interests: No competing interests were disclosed.
Is the topic of the opinion article discussed accurately in the context of the current literature?
Partly
Are all factual statements correct and adequately supported by citations?
Yes
Are arguments sufficiently supported by evidence from the published literature?
Yes
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
Partly
References
1. Devarapalli P, Bhalke S, Dharmadhikari N, Mishra V, et al.: Induced Pluripotent Stem Cells: Inventors turning into competitors. Journal of Commercial Biotechnology. 2017; 22 (4). Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Patent law, Patent analytics, Genomics, Synthetic biology
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Stem cells, regenerative medicine, cancer, developmental biology
Is the topic of the opinion article discussed accurately in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Are arguments sufficiently supported by evidence from the published literature?
Partly
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
Yes
References
1. Takashima K, Morrison M, Minari J: Reflection on the enactment and impact of safety laws for regenerative medicine in Japan. Stem Cell Reports. 2021; 16 (6): 1425-1434 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Stem cells, regenerative medicine, cancer, developmental biology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 4 (revision) 07 Dec 23 |
read | |
|
Version 3 (revision) 18 Aug 23 |
read | |
|
Version 2 (revision) 03 Jan 23 |
read | read |
|
Version 1 06 Sep 22 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)