Keywords
Staphylococcus aureus, Virulence factor, pvl, eta
This article is included in the Genomics and Genetics gateway.
This article is included in the Emerging Diseases and Outbreaks gateway.
Staphylococcus aureus is well known to cause a multitude of clinical manifestations, from mild to severe bloodstream infections that could lead to death. Infections are common, either in community-acquired or hospital-acquired settings, and treatment remains a challenge due to methicillin-resistant Staphylococcus aureus (MRSA). The pathogenesis of S. aureus is mediated by several cell-surface and secreted virulence factors. The virulence factors discussed in this study are Panton-Valentine leucocidin (pvl) and exfoliative toxin A (eta). Identifying both pvl and eta gene may help in studying bacterial pathogenesis and biology thus creating possible therapeutic pathway or intervention.
Our pilot study aimed to observe pvl and eta as virulence gene prevalence in a North Sumatera tertiary referral health center.
Our study was a descriptive-analytical observational study with a cross-sectional design in which we collected isolates over a single time period. The frequency of genes is reported as a percentage comparison between MRSA and methicillin-susceptible S. aureus (MSSA). Qualitative gene prevalence analysis was carried out using the polymerase chain reaction (PCR).
Our results showed that from 38 MRSA sample isolates, 32 samples were found to be pvl-positive, or 84,3% of the total samples. From 40 MSSA sample isolates, one sample was found to be pvl-positive MSSA, or 97,5%. Regarding eta, from 38 MRSA sample isolates, 81,6% of the total sample did not have eta, while from 40 MSSA sample isolates, all samples were found to be positive for eta. We found that both pvl and eta were significantly more likely to be expressed in the MSSA strain.
Our study shows that pvl and eta are more likely expressed in MSSA strains than in MRSA strains in Indonesia.
Staphylococcus aureus, Virulence factor, pvl, eta
We changed all “methicillin-sensitive” to “susceptible”.
We changed all “pvl” and “eta” to italic form.
Study design: "Sample lab numbers with MRSA and MSSA..." is not understandable. We had to rephrase this sentence.
We had deleted any redundant test that while performed in this study, wasn't necessary to proof Staphylococci existence (oxidase test etc).
We used MSA in this study not blood agar. Blood Agar, however, was used in the hospital clinical installation. This was changed in the new version.
We used VITEK-2 system in this study, Kirby Bauer method were not used because all result were consistent with mec gene identification.
All methods has been cited accordingly.
We had mention positive results instead of negative results.
We had given several suggestion for further studies in the end of limitations.
See the authors' detailed response to the review by Dewi Santosaningsih
See the authors' detailed response to the review by Amin Soebandrio
Staphylococcus aureus are Gram-positive spherical bacteria, usually arranged in a grape-like manner. This bacterium is well known to cause a multitude of clinical manifestations, from mild to severe bloodstream infections that could lead to death. Infections are common, either in community-acquired or hospital-acquired settings, and treatment remains a challenge due to multi-drug-resistant strains such as methicillin-resistant Staphylococcus aureus (MRSA).1 S. aureus, including MRSA, is commonly found on the skin and mucous membranes as part of the normal flora of human bodies.2 While usually known as a commensal bacterium, research has shown that S. aureus infection is one of the most prevalent in the world. In the industrialized world, Tong et al.’s review showed a 10 to 30 per 100,000 person-year incidence of bacteremia caused by S. aureus. While overall rates may have stabilized due to the rise of antibiotics, the contribution of MRSA has fluctuated.3 However, while it is well observed that staphylococcal infections caused by MRSA remain varying, in which at a specific time and location MRSA prevalence might be higher while in other time and location MRSA prevalence could be lower than predicted, studies have shown an increasing amount of MRSA infections in each decade. Hasanpour et al.’s research showed that before 2000, only 441 elderlies were infected with MRSA; however, this number skyrocketed to 4,365 in the 2001–2010 period and 11,987 in 2011–2022.4 As such, it can be said that MRSA creates a new challenge for healthcare workers and researchers to identify its pathogenesis and thus create a sound and reliable solution.
The pathogenesis of S. aureus is mediated by several cell-surface and secreted virulence factors. One such virulence factor is Panton-Valentine leucocidin (pvl).5 pvl is a toxin comprising two components, LukS-PV and LukF-PV. After their secretion, both components assemble into a pore-forming heptamer on neutrophil membranes, causing neutrophil lysis. There is a significant amount of research that shows the role of pvl in pathogenesis; however, it remains unclear what the trends are for pvl-positive methicillin- sensitive S. aureus (MSSA) or MRSA. The molecular epidemiology and burden of pvl-positive MSSA or MRSA are highly variable within studies, with the US dominated by pvl-positive MRSA, while such bacteria have been found to be rare in Australia, which has a predominance of both pvl-positive MRSA and MSSA.6 Prudent research toward pvl molecular epidemiology is vital because it is well known that pvl is associated with invasive disease and thus could be used as a gene marker for severe infection. In industrialized countries, such epidemiological studies have led to public health measures aimed at individuals infected with the pvl-producing strain.7
Another such virulence factor was identified as exfoliative toxin A (eta). Exfoliative toxins (ETs), also known as epidermolytic toxins, are serine proteases secreted by S. aureus that recognize and hydrolyze desmosome proteins in the skin. ETs have been associated with the loss of keratinocytes and cell-cell adhesion, inducing peeling of the skin and blister formation. One of the principal isoforms of exotoxins implicated in human skin damage is eta. Recognizing eta prevalence could help distinguish the extent of the damage caused by S. aureus.8 Research has shown that eta is a prevalent toxin. A study done by Mohseni et al. revealed that 76.7% of isolates were positive for eta in S. aureus isolates.9 However, research is limited toward MSSA, and there is a lack of research assessing eta prevalence in MRSA strains.
Due to the need for molecular epidemiological study in defining public health measures and mapping molecular profiles, especially in Indonesia and possibly in Southeast Asia, our study aimed to measure pvl and eta gene molecular prevalence at the Adam Malik General Hospital, one of Indonesia’s main tertiary referral health centers. Identifying both pvl and eta gene may help in studying bacterial pathogenesis and biology thus creating possible therapeutic pathway or intervention.
Our study was a descriptive-analytical observational study with a cross-sectional design, in which we collected isolates from the North Sumatera Tertiary Referral Center over a single time point which was in January 2022. Our study was carried out after it had been approved by the ethical medical research committee of the Faculty of Medicine, Universitas Sumatera Utara, Medan, Indonesia through letter no. 540/KEPK/USU/2022. Isolates were collected in a blind manner. This method was used to prevent bias in clinical correlation due to our specific study to identify molecular epidemiology at a specific time point, not to explain a phenomenon caused by a toxin or virulence factor specifically. We determined the sample size needed using the following descriptive sample formula:
To obtain 95% confidence interval, Za was pre-determined with 1.96 while d was 0.1. According to Mohseni’s study,8 which found P (proportion of eta positive sample in population) to be 0.767, we found that our study needed at least a total sample of 68 isolates to obtain statistical power. Our study included samples that were as evenly divided into MRSA and MSSA as possible.
After we obtained the minimal sample needed, we collected isolates from the Adam Malik General Hospital Microbiology Installation using cluster random sampling. All MRSA and MSSA samples within time periods were assigned a special code which then would be processed with computer randomization tool. We obtained 38 MRSA samples and 40 MSSA samples without information regarding sample clinical history to prevent bias. All samples were collected from bacteremia patient through blood collection. Colonies suspected to be S. aureus were cultured in mannitol salt agar (MSA) medium, while antibiotic susceptibility tests were performed with VITEK-2 system. Suspension obtained from blood agar medium was transferred to the Eppendorf and stored at -80°C. Suspension were made by combining 10 mL of aquabidest added with bacteria colony obtained from agar and then vortexed and inserted into densitometer.
MRSA or MSSA interpretation can be done with VITEK-2 system interpretation.
DNA extraction was done from bacterial cells using Presto Mini gDNA Bacteria (Geneaid). 1 × 109 colonies of bacterial cells were inserted in a 1.5 mL sterile tube and centrifuged at 13.000 rpm for 1 minute; afterwards, the supernatant was removed. Inside the tube, 200 μL of phosphate buffer saline (including 0.8 mL/200 μL lysozyme) were vortexed. Incubation was then performed at 20°C for 5 minutes, and then 20μL of proteinase K was added and vortexed. Incubations were then performed at 70°C for 10 minutes, then the result was added to 200 μL of absolute ethanol and vortexed. GD columns were then arranged inside the collection tube, and 400 μL of buffer W1 were centrifuged at 13,000 rpm for about 30 seconds. Then, liquids were extracted, and centrifugation was re-done with 600 μL wash buffer. After 100 μL of elution buffer were added and maintained at room temperature for 3 minutes, the tubes were filled with pure DNA. Tubes were saved in a freezer at -20°C. Our protocol was in accordance with GeneAid protocol specified inside the product kit.
In the PCR detection of the pvl and eta genes, specific primers were used after searching through the NCBI search engine to ensure their specificity. Table 1 lists the specifications for the primers used, and Table 2 describes our temperature program and volume.
| Name | Sequence | Amplitude size | Source publication |
|---|---|---|---|
| eta forward | 5′-TTTGCTTTCTTGATTTGGATTC-3′ | 464 bp | Mohseni et al., 20189 |
| eta reverse | 5′-GATGTGTTCGGTTTGATTGAC-3′ | ||
| pvl forward | 5′-ACAAGCAAAAGAATACAGCG-3′ | 575 bp | Hesari et al., 201810 |
| pvl reverse | 5′-GTTTTTGGCTGCTTCTCTTG-3′ |
Afterward, we began PCR mastermix preparation by liquefying GoTaq Green Master Mix 2×, primers (forward and reverse), nuclease-free water and DNA template. Our primer was obtained from Integrated DNA Technologies. These were then vortexed and spun down for 10 seconds, and a PCR mix was then prepared with the materials specified below (Table 2) to get one sample. Afterward, vortexing was done to ensure suspension homogeneity. DNA templates were then added, and the thermal cycling was carried out with the parameters listed in Table 2. In summary, for most steps, 30 seconds are sufficient, while in the first step, a 5-minute hot start at 94°C would be necessary. To detect pvl, 35 cycles consisting of 94, 52, and 72°C of denaturation, annealing, and extension were performed for 30 seconds. eta followed a similar step. Our protocol was in accordance with GeneAid protocol specified inside the product kit.
PCR products were electrophoresed using 2% agarose gel (Merck, Germany). A mixture of 1 λ DNA loading dye and 5 λ PCR product was loaded in the gel. Electrophoresis was performed at a voltage of 80 V. Our electrophoresis method was done in accordance with Merck instruction.
The electrophoresis results were obtained and visualized through a UV transilluminator. PCR results were defined as positive when a contrast streak was within the primer base pair or negative when the chamber was found to be empty after visualization. As the aim of the study was to identify specific virulence gene prevalences, quantitative PCR or RNA sampling were not performed. Visualization and analysis was done in accordance with Merck instruction and default software provided by electrophoresis tool.
SPSS version 25 and Microsoft Office Excel were used to analyze the data. The frequency of genes was reported as a percentage frequency, and a descriptive comparison was made between MRSA strain gene prevalence and that of MSSA. A bivariate analysis was performed to determine the statistical difference between groups. Due to the categorical nature of our data, the Chi-square test was chosen. Significance was proven with a p-value below 0.05 in the 95% confidence interval.
Our results showed that from 38 MRSA sample isolates, 32 samples were found to be pvl-positive, or 84.3% of the total samples. From 40 MSSA sample isolates, one sample was found to be pvl-positive MSSA, or 97.5%. Regarding eta, from 38 MRSA sample isolates, 81.6% of the total sample did not have eta, while from 40 MSSA sample isolates, all samples were found to be positive for eta. Afterwards, we analyzed our data with the Chi-square test to determine whether significant differences existed between pvl and eta prevalence. We found that both pvl and eta were significantly more likely to be present in the MSSA strain, with p ≤ 0.05, respectively. The results can be seen in Table 3.
| Variables | Total samples (n = 78) | MRSA (n = 38) | MSSA (n = 40) | p-value |
|---|---|---|---|---|
| pvl positive | 71 (91.1%) | 32 (84.3%) | 39 (97.5%) | 0.040* |
| pvl negative | 7 (8.9%) | 6 (15.7%) | 1 (2.5%) | |
| eta positive | 71 (91.1%) | 31 (81.6%) | 40 (100%) | 0.004* |
| eta negative | 7 (8.9%) | 7 (18.4%) | 0 (0%) |
Electrophoresis visualisation for each group can be seen in Figure 1 below.
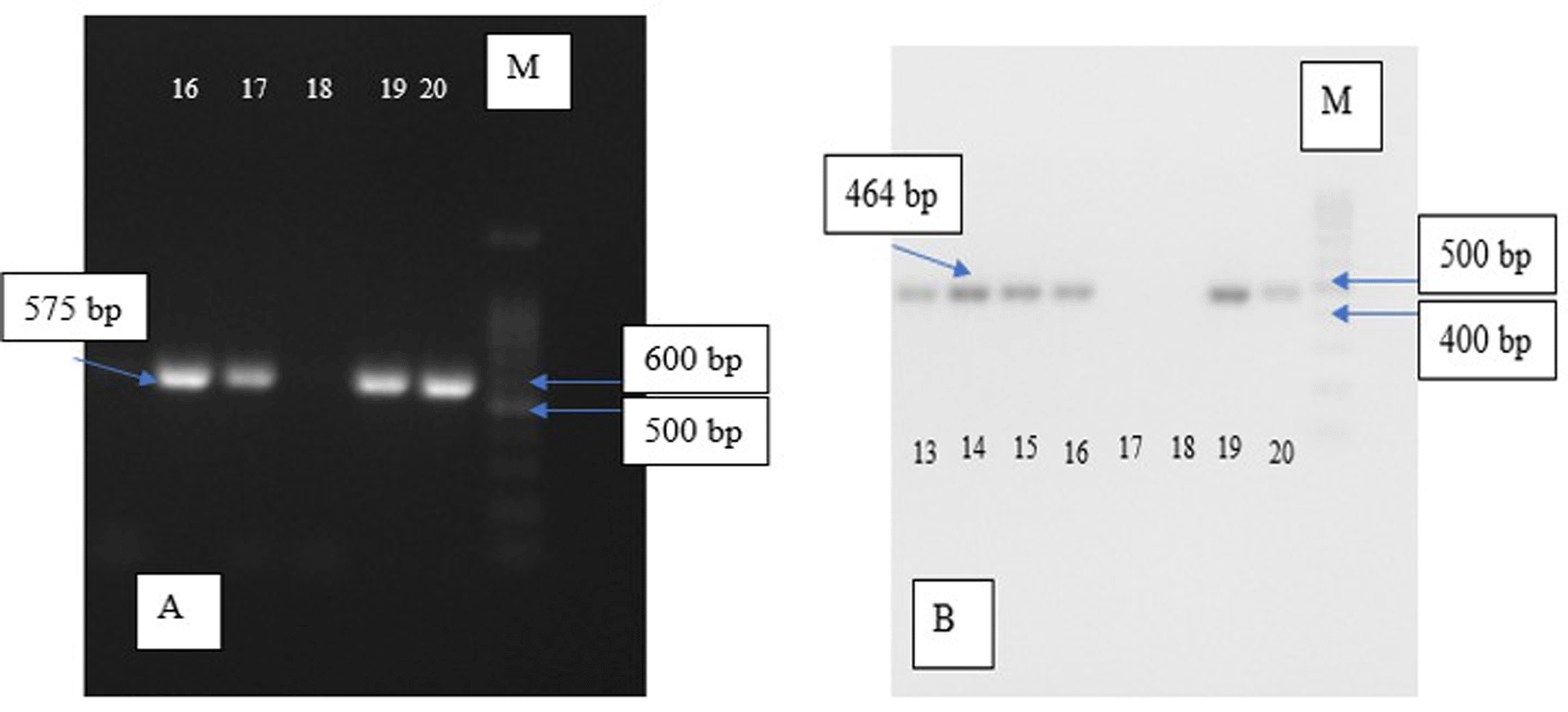
Our research was performed to identify the molecular epidemiology of pvl-positive MRSA, pvl-positive MSSA, and eta prevalence in either MRSA or MSSA, thus supporting local public health authorities in creating a sound public health measure and fulfilling data needs regarding S. aureus molecular epidemiology, especially in Southeast Asia. According to our results, most MRSA and MSSA were found positive for pvl and eta expression. We also found that MSSA was significantly more likely to encode both pvl and eta.
Our research shows very concerning new updates which revealed high pvl (+) prevalence in bacteremia patient. In a study performed by Boan et al. in Australia during the 2007–2009 period, pvl-negative and positive samples were still equally dominating, with 141 pvl-positive S. aureus and 148 pvl-negative strains. The Boan study did find that PVL didn’t significantly affect methicillin resistance, with 56% of pvl-positive strains found to be MRSA. However, it is interesting to note that in the Boan study, which reflects Australia’s S. aureus molecular epidemiology, it was shown that pvl-positive MRSA had begun to dominate in Western Australia.11 These results concur with our study, and by reflecting its close geographical distance, our result creates a new concern that pvl-positive MRSA and MSSA dominate most S. aureus specimens.
While all of our samples were procured from bacteremia patient, it might be interesting to analyzed source of infection in pvl (+) isolates. Melles research in 2006 point out high percentage of pvl (+) S. aureus in soft tissue infection isolates. This was not consistent with our finding however Melles research were performed nearly two decades before and changes in bacterial biology could happen within the span of time.
Recent research performed in Gambia by Darboe et al. in 2019 revealed a somewhat different result at several time points, while at the same time reflecting the similar results found in our study in another time points. While it is correct to assume that the pvl-positive samples have increased from 2005 levels to 2015 levels, their numbers has been fluctuating at best. However, it is interesting to note that in one period of the study, the pvl-positive strain was as high as 90%. Darboe’s study didn’t elaborate on whether there is methicillin resistance in pvl-positive or pvl-negative strains; however, Darboe did conclude that there was no association between pvl and antimicrobial resistance, while emphasizing the still-low antimicrobial resistance in Gambia.12 This result brought a new perspective to our study as it showed a fluctuating trend. It might be possible that some measures were taken during the lower pvl-positive strain infection period, and it has been found that while our results show unusually high pvl-positive MRSA and MSSA, this result was not irreversible.
Jaiswal’s report in 2022 revealed another different result. Their study results were not consistent with ours, whereby Jaiswal revealed only 49 out of 162 positive pvl strains within MRSA.13 We noted that the Jaiswal study was not consistent with our study or with other studies performed in India. A study by Kaur from India reported 85.1% pvl-positive MRSA prevalence within the country,14 similarly to D’Souza’s study in Mumbai which revealed 64% pvl-positive MRSA.15 This doesn’t necessarily mean that the Jaiswal study was incorrect, as it emphasized the dynamic change in MRSA genetic epidemiology. In a very large country like India, even molecular epidemiology could differ between regions and provinces.
Local studies at Andalas University performed by Linosefa et al. revealed a more balanced number. Linosefa found only two out of 19 samples to be positive for pvl.16 While this result was encouraging, it should be noted that this research was performed nearly a decade ago, and this research recommends the importance of surveillance, as was done in our study. This result also strengthened our hypothesis regarding how varying pvl prevalence could be.
Another interesting is the research done by Bhatta et al. in 2016 in Nepal. In his study, Bhatta observed that 90.4% of MRSA acquired in the community was pvl-positive, while pvl detected in nosocomial infections was only 7.1% positive. It was also found that pvls were not associated with bloodstream infections.17 While the number of samples makes it hard to draw any definitive scientific conclusion, there is a high possibility that Indonesian S. aureus-infected patients mostly acquire their infection from the community. Interventions toward personal hygiene or public health measures might indeed lower pvl-positive prevalence.
With regards to eta, our results also show a disturbing new discovery in which most MRSA and MSSA were positive for the eta gene, with MSSA being significantly more likely to have eta. However, this has actually been predicted by several studies. Mohseni, in his study conducted in Iraq, found that 87.3% of isolates, or 131 samples, were positive for at least ET genes, with eta being dominant. eta was found in 76.7% of the samples obtained by Mehsani. In the conclusion, Mehsani deemed the finding a serious problem as it may spread through gene transfer between strains.9
A study performed by Koosha et al. in 2013 is, however, quite consistent with our study. In the Kooesha study, lack of both eta and etb genes was only detected in eight (4%) isolates, and eta dominated ET prevalence. ETs play a role in colonization and invasion of injured mucosa and skin. Koosha also found that the distribution of ETs was largely similar between the MRSA and MSSA groups. Finally, drug resistance was abundant in the analyzed population.18 However, a study performed by Montazeri et al. in 2021 revealed that eta expression was only 23.7%. It must be emphasized that the Montazeri study was limited to cancer patients, who may have immunological disorders that could affect staphylococcal infection prevalence.19 This result might also emphasize regional fluctuations in molecular epidemiology. The study from the Middle East showed an even more surprising result: none of the MRSA isolates expressed the eta gene, out of 76 Iraqi isolates and 49 refugee isolates.20
The study conducted in the People’s Republic of China by Li et al. showed much more consistent results compared with our study. Li found that most MRSA and MSSA contained eta genes at rates of 61.8% and 55%, respectively. Li’s research compared the prevalence from year to year, and there was indeed an increasing prevalence of eta gene prevalence, which was 23.1% in the 2013–2014 period and increased to 80.1% in the 2018–2019 period.21 Our results revealed that Li’s increasing trend is persisting even in Indonesia.
A systematic review was conducted regarding eta-positive S. aureus in Iran alone. It showed quite variable results. Fooladi’s study showed 92.7% Staphylococcus aureus were positive for eta.22 On the other side of the spectrum, eta detection could be as low as 0%, as shown in the Rahimi study in 2018.23 This systematic review showed that even between regions, the variability of the S. aureus virulence gene is quite large.24
Lastly, a study in Indonesia done by Santosaningsih et al. in 2017 showed that only 11.3% of patients encoded eta gene.25 This result shows the importance of our study due to the very variable and volatile molecular epidemiology of S. aureus. Modifiable factors can help reduce the burden of disease.
The limitation of our study was the blind setting. While blind research might be ideal to recognize molecular epidemiology while preventing specific clinical bias (e.g., lower pvl prevalence in bloodstream infection),17 a clinical-to-molecular genetics relationship study could show the magnitude of this effect. Other limitations include our single-center study. However, our hospital is the main tertiary referral center in Sumatera Island, so it could reflect Staphylococcal molecular epidemiology in all of Sumatera. It still must be emphasized that most infections occur in the acute phase, and milder strains of S. aureus might have been treated in a primary health center. Another limitation is the use of bacteremia patient, further research should attempt to describe the prevalence of pvl and eta genes among either MSSA or MRSA clinical isolates based on the type of specimen. Through this method, severity of disease may be assessed. We proposed further research with double-blind, multi-center and clinical studies to elaborate the existing body of knowledge explained either in this study or in other studies.
Our study shows that pvl and eta are more likely expressed in MSSA strains than in MRSA strains in Indonesia. This is a relatively new finding and could have significant implications for treating MSSA, as pvl and eta have been deemed virulence factors that could worsen disease progression. Public health measures are necessary, and continued surveillance is important to ensure that pvl or eta prevalence as a virulence factor decreases with time and effort.
Figshare: Electrophoresis result of Staphylococcus aureus clinical isolates from Adam Malik General Hospital, https://doi.org/10.6084/m9.figshare.22796012.v1. 26
Figshare: Result of ETA and PVL PCR and Cefoxitime Screening of Clinical Isolates, https://doi.org/10.6084/m9.figshare.22849361.v1. 27
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Antimicrobial resistance; infection control; Staphylococcus aureus
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Antimicrobial resistance; infection control; Staphylococcus aureus
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical Microbiology, Immunology, Molecular Biology.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Melles D, van Leeuwen W, Boelens H, Peeters J, et al.: Panton-Valentine Leukocidin Genes inStaphylococcus aureus. Emerging Infectious Diseases. 2006; 12 (7): 1174-1175 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical Microbiology, Immunology, Molecular Biology.
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Antimicrobial resistance; infection control; Staphylococcus aureus
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 22 Feb 24 |
read | |
|
Version 2 (revision) 11 Jan 24 |
read | read |
|
Version 1 18 Aug 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)