Keywords
circadian clock, cancer, night shift work, early-onset cancer, colorectal cancer, Wnt signaling, chronotherapy, chronomedicine
This article is included in the Cell & Molecular Biology gateway.
This article is included in the Oncology gateway.
This article is included in the Circadian Clocks in Health and Disease collection.
In modern society, there is a growing population affected by circadian clock disruption through night shift work, artificial light-at-night exposure, and erratic eating patterns. Concurrently, the rate of cancer incidence in individuals under the age of 50 is increasing at an alarming rate, and though the precise risk factors remain undefined, the potential links between circadian clock deregulation and young-onset cancers is compelling. To explore the complex biological functions of the clock, this review will first provide a framework for the mammalian circadian clock in regulating critical cellular processes including cell cycle control, DNA damage response, DNA repair, and immunity under conditions of physiological homeostasis. Additionally, this review will deconvolute the role of the circadian clock in cancer, citing divergent evidence suggesting tissue-specific roles of the biological pacemaker in cancer types such as breast, lung, colorectal, and hepatocellular carcinoma. Recent evidence has emerged regarding the role of the clock in the intestinal epithelium, as well as new insights into how genetic and environmental disruption of the clock is linked with colorectal cancer, and the molecular underpinnings of these findings will be discussed. To place these findings within a context and framework that can be applied towards human health, a focus on how the circadian clock can be leveraged for cancer prevention and chronomedicine-based therapies will be outlined.
circadian clock, cancer, night shift work, early-onset cancer, colorectal cancer, Wnt signaling, chronotherapy, chronomedicine
We have made minor revisions and clarifications to this revised version to address reviewer comments . Specifically, we attempted to
See the authors' detailed response to the review by Kristin Eckel-Mahan
See the authors' detailed response to the review by Phillip Karpowicz
Biological rhythms regulate daily, seasonal, and long-term oscillations that are essential for life on Earth. The circadian clock is an evolutionarily conserved pacemaker found in prokaryotes and eukaryotes that governs homeostatic circuits that are fundamentally required for host fitness and survival. The mammalian clock is functionally conserved to regulate sleep/wake cycles (Czeisler et al., 1980; Winfree, 1983), feeding/fasting rhythms (Damiola et al., 2000; Hara et al., 2001; Inoue et al., 1977; Stokkan et al., 2001; Vollmers et al., 2009), and a host of endocrine, metabolic, and immune functions (Green et al., 2008; Keller et al., 2009; Kitchen et al., 2020; Turek et al., 2005). The focus of this review is on mammalian clocks and their roles in health and disease, with a particular focus on clocks in healthy versus transformed cells. Recent evidence has cited multiple diverse and tissue-specific functions of the circadian clock in different cancer types such as lung, colorectal, hepatocellular, breast, and others (Chun, Fortin, Fellows et al., 2022; Dong et al., 2019; Janich et al., 2011; Lee et al., 2010; Papagiannakopoulos et al., 2016; Puram et al., 2016; Stokes et al., 2021).
Notably, circadian regulation can occur not only at the level of the transcriptional and translational feedback loop (TTFL), but also through protein-based mechanisms (Gotoh et al., 2014; Huber et al., 2016). This review will provide a comprehensive overview of the divergent functions of the clock in cell cycle control, maintenance of genome integrity, and immunity in healthy tissues, in an attempt to deconvolute the elaborate cellular networks that the biological pacemaker impinges on. Additionally, the reported role of the circadian clock in different cancer types will be reviewed in the context of clinical and epidemiology data, pre-clinical in vivo mouse models, as well as mechanistic data from cell line-based studies. By considering the tissue- and cell-specific roles of the circadian clock as transcriptional regulators as well as at the protein level, this review will provide a comprehensive and updated understanding of the intriguing connections between the circadian clock and cancer biology.
The circadian clock is the internal biological pacemaker that controls cell autonomous 24-hour oscillations in gene expression programs that regulate organismal physiology ( Figure 1). The central clock, which resides in the suprachiasmatic nucleus (SCN) of the hypothalamus, is responsive to photic cues and transmits autonomic and endocrine signals to synchronize tissue-specific peripheral clocks to the environmental light-dark cycle (Pando et al., 2002; Welsh et al., 2004, 2010; Whitmore et al., 2000; Yamazaki et al., 2000). Peripheral clocks are also entrained by external cues including temperature (Barrett & Takahashi, 1995; Brown et al., 2002; Gould et al., 2006; Huang et al., 1995; Ruoff et al., 2005) and food supply that serve to further fine-tune biological timekeeping (Damiola et al., 2000; Hara et al., 2001; Inoue et al., 1977; Stokkan et al., 2001; Vollmers et al., 2009). The circadian system is regulated by a tightly controlled TTFL that encompasses a 24-hour day. The positive transcriptional activators of the circadian machinery, CLOCK and BMAL1, heterodimerize and bind to consensus E-box motifs located within promoters of core clock and clock-controlled genes (Ripperger & Schibler, 2006; Ueda et al., 2005). The core clock regulators of the negative arm of this TTFL, PERIOD (PER) and CRYPTOCHROME (CRY), are translated to repress the transcriptional activity of the CLOCK-BMAL1 complex (Duong et al., 2011; Michael et al., 2017; Nangle et al., 2014; Narasimamurthy et al., 2018). This entire transcriptional/translational feedback circuit drives the rhythmic periodicity of gene expression networks that govern endocrine function, metabolism, immune response, cell cycle control, and genome stability, many of which will be discussed below. Given the complex role of the clock in cellular metabolic control and nutritional challenge, and the host of recent publications covering this topic (Guan & Lazar, 2021; Reinke & Asher, 2019; Rijo-Ferreira & Takahashi, 2019; Verlande & Masri, 2019), this review will only cover clock-controlled metabolic alterations in the context of cancer.

In mammals, circadian rhythms are coordinated by the central circadian clock, located in the suprachiasmatic nucleus (SCN) of the hypothalamus (Inouye & Kawamura, 1979; Stephan & Zucker, 1972). The central clock receives photic cues and transmits endocrine and autonomic signals to synchronize tissue-specific peripheral clocks to the time of day (Pando et al., 2002; Welsh et al., 2004, 2010; Whitmore et al., 2000; Yamazaki et al., 2000). The circadian clock is regulated by a TTFL where CLOCK and BMAL1 drive transcriptional activation and PERIOD (PER) and CYPTOCHROME (CRY) feedback to repress this transcriptional activity. This TTFL regulates gene expression programs that modulate critical cellular processes needed to maintain homeostasis including cell division, maintenance of genome integrity, immunity, endocrine and metabolic functions. The circadian clock is implicated in regulating the growth and division of cells as the expression of cyclins is rhythmic (Graña & Reddy, 1995; Vermeulen et al., 2003). Circadian proteins also mediate the DNA damage response (Gery et al., 2006; Kang & Leem, 2014) and DNA repair including nucleotide excision repair (Gaddameedhi et al., 2011; Marteijn et al., 2014), base excision repair (Kozmin et al., 2005; Krokan & Bjørås, 2013), homologous recombination and non-homologous end-joining (Cotta-Ramusino et al., 2011; Shafi et al., 2021). Importantly, in addition to its transcriptional regulation, the circadian clock also exerts its function at the protein-level, with PER2 directly binding to inhibit p53 degradation (Gotoh et al., 2014) and CRY2 promoting MYC degradation (Huber et al., 2016). In addition to regulation of cell division and DNA damage, the immune system is also tightly regulated by the circadian clock to promote efficient immunologic response to infection. Immune cells have functional circadian clocks (Keller et al., 2009; Silver et al., 2012) and the release of cytokines and chemokines is rhythmic (Gibbs et al., 2014; Pariollaud et al., 2018), as well as the release of immune cells into the bloodstream (Dimitrov et al., 2007; Méndez-Ferrer et al., 2008). This rhythmic secretion of chemokines facilitates time of day trafficking of immune cells into tissues (Gibbs et al., 2014; Méndez-Ferrer et al., 2008) which has been demonstrated to mediate the host response to infection (Kiessling et al., 2017) and disease (Gibbs et al., 2014; Kitchen et al., 2020). Lastly, metabolic processes, including glucose and lipid metabolism, cardiovascular health and endocrine hormone secretion are regulated by the circadian clock (Green et al., 2008; Verlande & Masri, 2019). Figure created using BioRender.
Cells regulate their growth and division using cell cycle checkpoints, which ensure timely progression of the cell cycle under normal physiological conditions, and also halt cell cycle progression in instances of DNA damage, erroneous mitosis, or environmental stressors (Collins et al., 1997; Kastan & Bartek, 2004). The duration and transition of each cell cycle phase is orchestrated by the activation of specific cyclin-dependent kinases (CDKs) by their respective cyclins (Graña & Reddy, 1995; Vermeulen et al., 2003). Whereas expression of CDKs remains relatively constant throughout the cell cycle, each cyclin peaks in expression in a staggered and coordinated manner to drive the cell cycle with appropriate timing (Graña & Reddy, 1995; Vermeulen et al., 2003). Given the rhythmic nature of both the cell cycle and the circadian clock, several studies have worked to investigate and define the connections between these two systems. It has been suggested that circadian rhythms and the cell cycle are tightly phase-coupled and oscillate with a 1:1 frequency in mouse fibroblasts (Feillet et al., 2014), and this synchronization has even been shown at the single cell level in mammalian NIH3T3 fibroblasts (Bieler et al., 2014). More recently, data suggests that the cell cycle and the circadian clock can synchronize each other bidirectionally in mammalian systems (Yan & Goldbeter, 2019). However, the extent of this coupling is still not well understood, as some studies report additional findings that the cell cycle and circadian clock can in fact operate independently, as demonstrated in Rat-1 fibroblasts as well as Lewis lung carcinoma cell lines (Yeom et al., 2010; Pendergast et al., 2010).
Given the intimate links between the circadian clock and the cell cycle, what are the molecular connections driving this interplay? Importantly, several studies have investigated connections between the circadian clock and the proto-oncogene MYC, a master regulator of cell cycle control that promotes cellular growth by driving cyclin expression and repressing CDK inhibitor activity (Burchett et al., 2021). In regard to transcriptional regulation of c-Myc , it has been reported that Bmal1-/- mice exhibited increased expression of c-Myc , whereas c-Myc expression was decreased in Cry1-/-;Cry2-/- mice (Liu et al., 2020), demonstrating a strong correlation between these networks. Additionally, MYC and NMYC can impinge on circadian rhythms in U2OS and SHEP cells, respectively, further highlighting the molecular crosstalk between the clock and the cell cycle that also integrates with cellular metabolic state (Altman et al., 2015; Shostak et al., 2016; Moreno-Smith et al., 2021). In regard to regulation of MYC protein levels, it was demonstrated that CRY2 mediates MYC degradation and that Cry2-/- knockout induced increased proliferation and transformation rates (Huber et al., 2016).
In addition to circadian links to MYC, clock-dependent transcription has been observed for many other cell cycle modulators, including cyclin D, cyclin E, cyclin A, and cyclin B in human epithelium (Bjarnason et al., 1999; Fu et al., 2002). Likewise, G2/M regulator WEE1, which inhibits Cyclin B1-CDK1 activity, has oscillating protein expression and kinase activity and this oscillation was dampened in Cry1-/-;Cry2-/- mice (Gréchez-Cassiau et al., 2008; Matsuo et al., 2003). Another study found that CLOCK and BMAL1 knockdown leads to the suppression of WEE1 and thus an increased activation of apoptosis in human hepatocellular carcinoma cell lines, again confirming circadian influence on WEE1 regulation (Qu et al., 2023). Additional CDK inhibitors such as p21cip1/waf1 and p16-Ink4A also oscillate under circadian control and this rhythmicity was lost in Bmal1-/- mice or Per1Brdm1/Brdm1;Per2Brdm1/Brdm1 mutant mice, respectively (Gréchez-Cassiau et al., 2008; Kowalska et al., 2013). Taken together, these data highlight the important molecular links between the circadian clock and cell cycle control mechanisms.
Due to a multitude of interactions between circadian proteins and cell cycle checkpoints and drivers, it is not surprising that circadian rhythm disruption can modify rates of cellular proliferation. For example, genetic clock disruption in mouse osteoblasts via Per1-/-;Per2m/m knockout or Cry1-/-;Cry2-/- knockout resulted in increased proliferation (Fu et al., 2005). Circadian clock disruption can potentially interfere with normal rates of cellular growth, introducing susceptibility to disease and cancer. Altogether, this data suggests that oscillations of clock proteins contribute to the proper expression of important cell cycle regulators that impact cellular proliferation.
Another important aspect of cell cycle regulation features the DNA damage response (DDR). Individual cells can receive tens of thousands of DNA lesions per day, which leads to replication errors, transcription blockage, and even permanent mutations if left unrepaired (Jackson & Bartek, 2009). Thus, the DDR has evolved to preserve genome integrity by recognizing various forms of DNA damage, stimulating DNA repair, inhibiting cell cycle progression until the repair is complete, and inducing apoptosis if the damage is irreparable (Giglia-Mari et al., 2011; Jackson & Bartek, 2009; Roos & Kaina, 2013).
In mammals, central DDR proteins ATR and ATM instigate the DDR by phosphorylating CHK1 and CHK2, respectively (Jackson & Bartek, 2009). Consequently, phosphorylated CHK1 and CHK2 activate transcription factor p53 to halt cell cycle progression (Ronco et al., 2017). It was reported that circadian proteins CRY1 and TIM modulate ATR and CHK1 activity in a time-of day dependent fashion, although it was found that Cry1-/-;Cry2-/- mouse embryonic fibroblasts (MEFs) still retained appropriate levels of checkpoint activity (Kang & Leem, 2014). Also, ATM and CHK2 form a complex with circadian protein PER1, and it was further demonstrated that PER1 knockdown reduced ATM-mediated CHK2 phosphorylation and dampened apoptotic response to DNA damage in human colon cancer cell line HCT116 (Gery et al., 2006).
Furthermore, a bi-directional crosstalk between the circadian clock and p53 has been reported. The p53 tumor suppressor plays a key role in stimulating DNA damage checkpoints and a circadian oscillation of p53 transcription has been reported in human oral epithelium (Bjarnason et al., 1999). Concurrently, it has been demonstrated that p53 modulates circadian activity in mice by directly binding to the promoter of Per2 and repressing Per2 expression (Miki et al., 2013). Moreover, PER2 directly binds to the C-terminal end of human p53 and slows MDM2-mediated degradation of p53 (Gotoh et al., 2014), indicating multiple points of interplay between these two mechanisms. The consequences of clock regulation on p53 were evident in Per2m/m mutant mice that exhibited lower levels of p53, increased resistance to p53-mediated apoptosis, and higher sensitivity to γ radiation (Fu et al., 2002). p53 activates the CDK inhibitor p21cip1/waf1 to induce cell cycle arrest (Al Bitar & Gali-Muhtasib, 2019). As stated in the previous section, p21cip1/waf1 expression oscillates under circadian control, further demonstrating clock regulation of the DDR. Interestingly, it was reported that either Cry1-/- or Cry2-/- MEFs exhibit altered expression patterns of the p21cip1/waf1 transcript, Cdkn1a, in response to genotoxic stress via doxorubicin (Papp et al., 2015). This study further demonstrated that genotoxic stress can shift the CRY1/CRY2 ratio and consequently change circadian period length (Papp et al., 2015), again demonstrating the crosstalk between the DDR and the molecular machinery of the circadian clock.
In summary, these studies suggest that circadian proteins exert a wide influence on proper cellular response to the daily insults of DNA damage. Impaired DDR via clock disruption increases the likelihood of cell proliferation despite unresolved mutations, which is a major contributor to cancer progression. Furthermore, since DDR proteins are commonly targeted during chemotherapy to inhibit rapidly dividing cells, understanding the effects of oscillating circadian proteins and their impact on the DDR may result in enhanced efficacy of cancer therapeutics.
The circadian clock not only plays a role in regulating DNA damage checkpoints, but also affects the ability of cells to perform DNA repair. Cells are equipped with multiple repair pathways that act to maintain DNA sequence fidelity following damage from endogenous and exogenous sources. Interestingly, while each DNA repair pathway has several components, the activities of certain key components exhibit striking transcriptional regulation through the circadian clock (Sancar et al., 2010).
Nucleotide excision repair (NER) removes bulky chemical adducts that distort the DNA helix, most importantly UV-induced intrastrand crosslinks (Marteijn et al., 2014). NER relies on the xeroderma pigmentosum group A (XPA) protein to recognize the lesion and coordinate incision and removal by the XPF-ERCC1 endonuclease complex (Marteijn et al., 2014). It was recently described that XPA oscillates in a circadian fashion, thus repair activity after UV irradiation also follows circadian rhythmicity. However, this rhythmicity was lost in Cry1-/-;Cry2-/- mice, clearly demonstrating that NER activity is regulated by the clock (Gaddameedhi et al., 2011). As sunlight is the major source of UV radiation, this connection between DNA repair of UV-induced damage and the circadian clock is intriguing.
Base excision repair (BER) uses a variety of different DNA glycosylases to remove damaged bases, leaving an abasic site that is subsequently processed by enzymes that carry out cleavage, gap-filling and ligation to restore DNA integrity (Krokan & Bjørås, 2013). The expression and activity of one such glycosylase, 8-Oxoguanine DNA glycosylase (OGG1), oscillates under clock control and OGG1 levels were disrupted in a human cohort performing shift work (Manzella et al., 2015). Importantly, this study was performed using lymphocytes collected from human blood whereas another study found that OGG1 does not oscillate in human keratinocytes (Hettwer et al., 2020). This suggests a potential cell-type specific role of OGG1 circadian regulation. As OGG1 recognizes a specific type of oxidative damage (i.e., 7,8-dihydro-8-oxoguanine opposite cytosine or thymine), it remains to be seen why this enzyme has evolved to be clock-controlled. One interesting link is that OGG1 has been shown to be required to prevent mutations induced by UVA (Kozmin et al., 2005), suggesting an additional role of the clock in repairing DNA damage following sunlight exposure.
DNA double strand breaks (DSBs) represent one of the most serious threats to genome integrity and multiple repair pathways have evolved for their repair, including homologous recombination (HR) and non-homologous end joining (NHEJ) (Chang et al., 2017; Sterrenberg et al., 2022). Due to the number of environmental and cellular sources of DSBs, understanding the role of the circadian clock in their repair is critical. Using HEK293T cells, CLOCK binding was found at several enhancer or transcriptional regulatory sites controlling DNA damage related genes including CDKN1A encoding p21, which mediates cell cycle arrest, as well as BRCA1 and RAD50, which play important roles in DSB repair (Alhopuro et al., 2010). Furthermore, CLOCK knockdown in human U2OS osteosarcoma cells resulted in abnormal cell cycle checkpoint response following irradiation and increased sensitivity to mitomycin C, indicative of a CLOCK-dependent response to repair DSBs (Cotta-Ramusino et al., 2011). CLOCK was also found to localize to laser-induced DSBs in U2OS cells, suggesting a potential direct role in the cellular signaling machinery required for DSB repair (Cotta-Ramusino et al., 2011). In addition to CLOCK, CRY1 is another circadian protein linked to DSB repair efficiency. CRY1 knockdown showed delayed DSB resolution in C4-2 and 22Rv1 cell cultures, and conversely, DSB resolution is enhanced upon treatment with the CRY1 stabilizer KL001 (Shafi et al., 2021). Transcriptomic analysis suggested that CRY1 regulated the expression of several major HR genes (including RAD51, BRCA1, and BRCA2) and other genes involved in NER, BER, mismatch repair (MMR), and NHEJ (Shafi et al., 2021). This study was specifically carried out in human prostate cancer cell lines as well as tissues from prostate cancer patients, and further studies are needed to define the role of CRY1 in regulation of DNA repair genes in other tissues, particularly those that are not hormone responsive.
Overall, these studies show that the effect of the circadian clock on DNA repair is widespread across multiple repair pathways. Although the mechanistic links continue to be investigated, current data suggests that robust circadian rhythms contribute to optimal genome integrity. Further understanding of how the circadian clock potentiates faithful DNA repair through multiple pathways is paramount for developing strategies to both prevent cancer, and to establish better and less toxic treatments for patients undergoing chemo- and radiation- therapy that acts through damaging DNA.
The goal of the immune system is to be primed to respond to insult through a complex network of different organs, proteins and pathways. It may be advantageous for immune parameters to cycle with activity of an organism, potentially allowing for the host to respond more efficiently to infection. In support of this, the circadian clock has been shown to regulate key parameters of immunity including cytokine release, immune cell number and trafficking, as well as the inflammatory response.
Immune cells, including splenic macrophages, dendritic cells (DCs), and B cells have been found to have cell-autonomous circadian clocks which directly control cellular immune function and timing (Keller et al., 2009; Silver et al., 2012). Cytokines and chemokines are small proteins that regulate the growth, activity and trafficking of immune cells and proper regulation of these proteins is essential for host immune defense. Importantly, the circadian clock has been linked to the production and release of cytokines and chemokines. Upon bacterial endotoxin stimulation, the secretion of TNFα and IL-6 by isolated ex vivo spleen derived macrophages was found to oscillate in a time-of-day dependent manner, including 8% of the macrophage transcriptome (Keller et al., 2009). Additional studies demonstrated an important role for Bmal1 in regulating cytokine response. Temporal gating of endotoxin-induced cytokine response in mice, a crucial feature of innate immunity, is dependent on the circadian clock as rhythmic gating of endotoxin response is lost in Bmal1-/- macrophages (Gibbs et al., 2012). This was found to be due to the suppression of Nr1d1, hereafter referred to as Rev-Erbα (Gibbs et al., 2012). An additional role of Bmal1 in regulating the immune response was identified with the genetic ablation of Bmal1 in bronchiolar cells that disrupted the rhythmic expression of the CXCL5 chemokine (Gibbs et al., 2014). These data suggest that the rhythmic release of cytokines is directly regulated by the circadian clock.
In addition to the rhythmic secretion of cytokines by immune cells, the circadian clock controls immune cell number and infiltration. For example, the number of hematopoietic stem cells (HSCs) and mature immune cells released from the bone marrow into the blood peaks at the beginning of the rest phase in mice (Méndez-Ferrer et al., 2008). In addition to the release of immune cells into the bloodstream, the circadian clock also modulates immune cell trafficking into tissues as evidenced by the rhythmic expression of CXCL5 and CXCL12 that regulate the trafficking and infiltration of neutrophils and HSCs, respectively (Gibbs et al., 2014; Méndez-Ferrer et al., 2008). Human studies provide additional evidence of circadian regulation of immune cell trafficking. Immune cells present in the blood of individuals on a normal sleep-wake cycle were compared to those on 24 hours of wakefulness (Dimitrov et al., 2007). It was found that the number of DCs and T cells in the blood is highly rhythmic and that sleep induced the expression of IL-12 which increased the number of monocytic DCs in the blood (Dimitrov et al., 2007). A more recent study found that individuals with blunted rest-activity rhythms exhibited increased inflammatory markers and elevated circulating white blood cells and neutrophils (Xu et al., 2022). These studies demonstrate a clock-controlled immune response through regulation of immune cell release into the bloodstream and trafficking into tissues.
Clock control of the immune system is critical for proper response to infection (Kiessling et al., 2017) and disease (Gibbs et al., 2014; Kitchen et al., 2020), and even vaccination (Cervantes-Silva et al., 2022). In support of this, mice infected with Salmonella enterica in the early rest period exhibited a high pathogen load and a stronger proinflammatory response (Bellet et al., 2013) and the magnitude of Leishmania parasitic infection in mice varied over 24 hours (Kiessling et al., 2017). These differences in infection and inflammation may be due to the time-dependent release of cytokines and immune cells. Indeed, the circadian expression of chemo attractants and the rhythmic infiltration of neutrophils and macrophages was lost in clock deficient macrophages (Kiessling et al., 2017; Sato et al., 2014). Additionally, pulmonary inflammation was found to be regulated by the rhythmic expression of the chemokine CXCL5 leading to time-of-day dependent neutrophil recruitment to the lung (Gibbs et al., 2014). Bmal1-/- bronchiolar cells lack this rhythmic CXCL5 expression leading to exaggerated inflammatory response and an impaired host response to Streptococcus pneumoniae infection (Gibbs et al., 2014). Bmal1 deletion suppresses Rev-erbα expression, and it was found that Rev-erbα-/- mice exhibit an exaggerated neutrophilic inflammatory response (Pariollaud et al., 2018). Furthermore, myeloid specific deletion of Bmal1 disrupts the diurnal trafficking of Ly6Hi inflammatory monocytes and promotes inflammation by inducing expression of monocyte attracting chemokines (Nguyen et al., 2013).
Overall, these studies establish the circadian clock as a critical regulator of the immune response through the release of cytokines and the trafficking of immune cells. This leads to a time-of-day dependent proinflammatory response to challenge such as bacterial or pathogenic infection. Moreover, disruption of the circadian clock has the potential to alter the daily rhythm of the immune system and lead to various types of diseases, including cancer.
In the previous section, we described how the circadian clock regulates critical cellular processes including cell cycle control, the DNA damage response and repair, as well as immunity. These processes are included as ‘Hallmarks of Cancer’ that are dysregulated during transformation (Hanahan, 2022; Hanahan & Weinberg, 2000, 2011), which suggests that the circadian clock may be involved in tumorigenesis. In support of this, we describe how the circadian clock is associated with cancer by looking at epidemiological data, early-onset cancers, and the tissue-specific and cell-type dependent function of the clock in various model systems.
About one quarter of the US population participates in night shift work (Alterman et al., 2013; Drake & Wright, 2017), which causes significant misalignment between the endogenous circadian clock and the sleep-wake cycle (James et al., 2017). Night shift work has been implicated as a risk factor for cancer and a systematic review of night shift work and cancer was recently reported (IARC, 2020). Several studies were identified that aimed to assess a correlation between night shift work and cancer risk, and these reports are summarized in Table 1. The most extensively studied association was between night shift work and breast cancer, and the majority of these reports found that night shift work increased the risk of developing breast cancer (Cordina-Duverger et al., 2018; Hansen & Lassen, 2012; Jones et al., 2019; Schernhammer et al., 2006). Several studies also noted an increased risk with duration of exposure and cumulative exposure to night shift work (Cordina-Duverger et al., 2018; Davis et al., 2001; Hansen & Lassen, 2012; Hansen & Stevens, 2012; Lie et al., 2011). In addition to breast cancer, shift work was also found to increase the risk of developing prostate, colon and rectum, lung, stomach, ovarian and pancreatic cancer. Although numerous studies cite a positive correlation between night shift work and cancer incidence, other studies report no effect (Jones et al., 2019; Koppes et al., 2014; Li et al., 2015; O’Leary et al., 2006; Pronk et al., 2010; Travis et al., 2016; Vistisen et al., 2017). There are multiple explanations for the contradictory data, including the lack of a standardized definition for night shift work, self-reporting collection process, and adjustment for confounding factors such as socioeconomic status and lifestyle. These limitations should be addressed and larger, more comprehensive studies are needed with multiple cancer types to define the epidemiological link between the circadian clock and cancer risk.
| Reference | Cancer type | Night shift work increases risk of cancer (yes/no) |
|---|---|---|
| (Tynes et al., 1996) | Breast | Yes, women over 50 |
| (Lie et al., 2006) | Breast | Yes, with 30+ years |
| (Pronk et al., 2010) | Breast | No |
| (Lie et al., 2011) | Breast | Yes, with 5+ years, risk increased with duration of exposure and cumulative exposure |
| (Hansen & Stevens, 2012) | Breast | Yes, risk increased with duration of exposure and cumulative exposure |
| (Hansen & Lassen, 2012) | Breast | Yes, risk increased with duration of exposure and cumulative exposure |
| (Knutsson et al., 2013) | Breast | Yes |
| (Koppes et al., 2014) | Breast | No |
| (Li et al., 2015) | Breast | No |
| (Åkerstedt et al., 2015) | Breast | Yes, with 20+ years |
| (Travis et al., 2016) | Breast | No |
| (Wegrzyn et al., 2017) | Breast | Yes, with over 20+ years |
| (Vistisen et al., 2017) | Breast | No |
| (Jones et al., 2019) | Breast | No |
| (Schernhammer et al., 2001) | Breast | Yes |
| (Schernhammer et al., 2006) | Breast | Yes |
| (Cordina-Duverger et al., 2018) | Breast | Yes, risk increased with duration of exposure and cumulative exposure |
| (Pesch et al., 2010) | Breast | Yes |
| (Rabstein et al., 2013) | Breast | Yes |
| (Fritschi et al., 2013) | Breast | Yes |
| (Grundy et al., 2013) | Breast | Yes |
| (Menegaux et al., 2012) | Breast | Yes |
| (Cordina-Duverger et al., 2016) | Breast | Yes |
| (Papantoniou et al., 2016) | Breast | Yes |
| (Davis et al., 2001) | Breast | Yes, risk increased with duration of exposure and cumulative exposure |
| (O’Leary et al., 2006) | Breast | No |
| (Wang et al., 2015) | Breast | Yes |
| (Yang et al., 2019) | Breast | Yes |
| (Barul et al., 2019) | Prostate | Yes |
| (Wendeu-Foyet et al., 2018) | Prostate | Yes |
| (Papantoniou et al., 2017) | Colorectal | Yes |
| (Schernhammer et al., 2003) | Colorectal | Yes |
| (Gu et al., 2015) | Lung | Yes |
| (Schernhammer et al., 2013) | Lung | Yes |
| (Gyarmati et al., 2016) | Stomach | Yes |
| (Carter et al., 2014) | Ovarian | Yes |
| (Parent et al., 2012) | Pancreatic | Yes |
The previous section highlighted the potential increase in cancer incidence in populations that participate in night shift work, which is known to disrupt circadian rhythms. However, there is mounting concern for circadian disruption in the general population as the access to technological devices continues to increase. Gradisar et al. demonstrated that nine out of 10 individuals surveyed use a technological device in the hour before bed, with the use increasing in individuals under 30 years of age (Gradisar et al., 2013). Among the Japanese population, young adults between the ages of 15 to 20, were exposed to the highest intensity of artificial light-at-night (Chen et al., 2022). The exposure to dim light at night through the use of devices has been shown to disrupt circadian rhythmicity by suppressing melatonin and impairing sleep quality (Lee & Kim, 2019). This suggests that younger individuals may be exposed to more environmental factors that disrupt the circadian clock than older populations. Strikingly, the average annual increase in the incidence of all cancers in young adults aged 15 to 39 years old has continued to increase since 1975 (Miller et al., 2020). A review of 98 articles published between 1995–2020 found that the incidence of colorectal, breast, kidney, pancreas, and uterine cancer is increasing in younger age groups (di Martino et al., 2022). In addition to the increasing incidence, studies have also suggested that the underlying biology of cancer in young adults differs from the same cancer in children or older individuals (Tricoli et al., 2016, 2018). Altogether, this introduces the idea that environmental circadian clock disruption in younger populations may contribute to the increasing incidence of early-onset cancers, though further studies are needed to confirm this experimentally.
It is worth noting that the increasing trends of early-onset cancers are strongest for colorectal cancer (CRC). Between 1975 and 2010, there has been a steady decline in CRC incidence rates in adults over the age of 50. However, in patients aged 20 to 34, the incidence of CRC has continued to rise (Bailey et al., 2015). If this trend continues, it is expected that by 2030, the incidence of colon and rectal cancer in individuals aged 20 to 34 will increase by 90% and 124.2%, respectively (Bailey et al., 2015). It was also identified that the increasing incidence of CRC in younger populations was greatest among Hispanics and African Americans, suggesting an alarming cancer health disparity (Augustus & Ellis, 2018; Muller et al., 2021; Singh et al., 2014).
Importantly, the intestine may be particularly sensitive to circadian disruption due to several reasons. The intestine is a highly regenerative organ, with complete cell renewal occurring every few days (Van Der Flier & Clevers, 2009). This constant renewal process is tightly regulated by the circadian clock, and disruption of circadian rhythms can affect the timing and coordination of this turnover process (Codoñer-Franch & Gombert, 2018; Stokes et al., 2017; Yoshida et al., 2015). The gut microbiota, which play a crucial role in digestion and immune function, also exhibit circadian rhythmicity and can be negatively impacted by circadian disruption (Heddes et al., 2022; Leone et al., 2015; Liang et al., 2015; Thaiss et al., 2016). Moreover, food intake is a powerful environmental cue synchronizing the circadian clock in peripheral tissues (Zarrinpar et al., 2014). Feeding mice only during the light phase, when mice are inactive, causes a phase shift in peripheral clocks of the liver, kidney, heart and pancreas (Damiola et al., 2000; Stokkan et al., 2001), demonstrating that the timing of food intake can disrupt the circadian clock. Nutritional challenge has also been shown to dynamically impact the circadian clock, including high fat diet and time-restricted eating (Acosta-Rodríguez et al., 2022; Chaix et al., 2019; Eckel-Mahan et al., 2013; Hatori et al., 2012). These studies establish the importance of coordinated cell renewal, gut microbiota, diet and timing of food intake in maintaining robust circadian rhythms in the intestine. It will be important to study the impact of alterations in these environmental and behavioral factors on the alarming increase in CRC rates in younger populations, as well as other cancer types.
Although there is mounting evidence suggesting that the circadian clock is implicated in various types of cancer, the mechanism underlying the role of the clock in cancer is still being uncovered. Table 2 outlines significant findings that provide clues for how the circadian clock functions in various cancer types and model systems. Based on these studies, the circadian clock has been implicated in both the initiation and the progression of cancer through the regulation of oncogenic pathways, cell cycle control, DNA damage repair, stemness, immunity and metastasis ( Figure 2). However, the effect of clock disruption on tumorigenesis may be tissue and model-specific. For example, knock out of the core clock gene BMAL1 in mouse models of solid tumors promotes tumor progression in CRC (Chun, Fortin, Fellows et al., 2022; Stokes et al., 2021), lung (Papagiannakopoulos et al., 2016), and other cancer types (Lee et al., 2010) but reduces the development of cutaneous squamous tumors (Janich et al., 2011). Furthermore, downregulation of BMAL1 in human glioblastoma stem cells halts their growth (Dong et al., 2019; Puram et al., 2016). As more studies are done in multiple cancer types and model systems, we may begin to better delineate the tissue-specific effects of clock disruption on cancer.
| Reference | Model system | Cancer type | Finding |
|---|---|---|---|
| (Shilts et al., 2018) | TCGA | Over 20 cancer types | Coordinated clock gene expression is lost in tumor vs non-tumor samples. |
| (Ye et al., 2018) | TCGA, cell lines | 32 cancer types | Clock genes are associated with activation/inhibition of oncogenic pathways, mutations in core clock genes correlated with patient survival, and circadian rhythmicity is lost in cancer cell lines. |
| (Wu et al., 2019) | TCGA | 11 cancer types | Core circadian clock genes are dysregulated in cancer and dysregulation correlated with poor patient prognosis and T cell exhaustion. |
| (Papagiannakopoulos et al., 2016) | Mouse | Lung | Genetic (Per2m/m and Bmal1-/-) and environmental (jet lag) clock disruption increased lung tumorigenesis in K-rasLSL-G12D/+;p53flox/flox or K-rasLSL-G12D/+ (K) mice. |
| (Pariollaud et al., 2022) | Mouse and human | Lung | Environmental clock disruption through jet lag increased lung tumor burden in KrasLSL-G12D/+ and enhanced HSF1 signaling. Inhibition of HSF1 reduced the growth of human lung cancer cells. |
| (Lee et al., 2010) | Mouse | Lymphoma, osteosarcoma, liver, angiosarcoma, ovarian, uterine | Per1-/-, Per2-/-, Cry1-/-, Cry2-/- and Bmal1-/- mice presented with increased spontaneous and radiation-induced tumor development. |
| (Kettner et al., 2016) | Mouse | Liver | Chronic jet lag induced spontaneous HCC in WT mice. |
| (Fu et al., 2002) | Mouse | Liver | Gamma irradiation of Per2-/- mice caused increased tumor development and reduced apoptosis in thymocytes. Genes involved in cell cycle regulation and tumor suppression were deregulated in Per2-/- mice. |
| (Wood et al., 2008) | Mouse | Colorectal | Per2-/- mice developed colonic poylps and ApcMin/+/Per2-/- developed significantly more intestinal polyps than ApcMin/+ mice. |
| (Stokes et al., 2021) | Mouse | Colorectal | ApcMin/+/Bmal1-/- mice developed more intestinal polyps than ApcMin/+ mice. |
| (Chun, Fortin, Fellows et al., 2022) | Mouse and human tumors ex vivo | Colorectal | Apcex1-15/Bmal1-/- mice developed more intestinal polyps than Apcex1-15 mice. Organoids from Apcex1-15/Bmal1-/- mice transformed into tumor spheroids due to Apc LOH. Circadian rhythms were lost in human colorectal tumors versus normal surrounding epithelial. |
| (Hadadi et al., 2020) | Mouse | Breast | Chronic jet lag increases cancer cell dissemination and lung metastasis, enhances stemness and promoted tumorigenesis by creating an immunosuppressive tumor microenvironment. |
| (Diamantopoulou et al., 2022) | Human, mouse | Breast | Intravasation of circulating breast tumor cells, which were prone to metastasize, occurred more frequently at night. |
| (Shafi et al., 2021) | In vitro, in vivo and human tumors ex vivo | Prostate | CRY1 expression is correlated to poor patient survival. CRY1 is stabilized by DNA damage in cancer and regulates homologous recombination. |
| (Chan et al., 2021) | TCGA and primary mouse fibroblasts | Bladder, colorectal, breast, stomach, melanoma, head and neck | CRY2 is mutated in human bladder, colorectal, breast, stomach, melanoma, and head and neck cancers. Cry2 mutation in MYC-transformed fibroblasts suppressed p53 gene expression and enhances growth. |
| (Fekry et al., 2018) | Mouse | Liver | Bmal1 expression in HNF4α-positive HCC prevented the growth of tumors in vivo. |
| (Sulli et al., 2018b) | Human in vitro and mouse in vivo | Multiple cancer types including colon, breast, melanoma and glioblastoma | Treatment of human cancer cells with SR9009, a REV-ERBα/β agonist, impaired viability and promoted apoptosis Treatment of glioblastoma in mice with SR9009 reduced growth, triggered apoptosis and improved survival |
| (Janich et al., 2011) | Mouse | Cutaneous squamous carcinoma | Bmal1-/-/K5-SOS mice developed fewer tumors than Bmal1+/+/K5-SOS mice. |
| (Dong et al., 2019) | Human ex vivo | Glioblastoma stem cells | Downregulating BMAL1 and CLOCK induced cell cycle arrest and apoptosis. Small molecule agonists targeting Cryptochromes and REV-ERBs downregulated stem cell factors and reduced GSC growth. |
| (Chen et al., 2020) | Mouse | Glioblastoma | CLOCK enhanced stem cell self-renewal and promoted protumor immunity through OLFM3 expression. CLOCK depletion in GSC272 and GSC20 tumors prior to implantation extended overall survival. |
| (Puram et al., 2016) | In vitro and in vivo | Acute myeloid leukemia | Inhibiting Bmal1 in AML cells reduced self-renewal. Bmal1-/- AML cells exhibited a growth defect compared to Bmal1+/+ AML cells. Irradiated WT mice transplanted with Bmal1-/- AML cells survived significantly longer than mice transplanted with Bmal1+/+ AML cells. |
| (Altman et al., 2015) | Cell lines | Neuroblastoma | Overexpression of Bmal1 suppressed colony formation. |
| (Moreno-Smith et al., 2021) | Cell lines | Neuroblastoma | MYCN suppressed BMAL1 expression to promote cell survival and this is attenuated by overexpression of BMAL1 by SR1078 treatment. |
| (Shostak et al., 2016) | Cell lines | Osteosarcoma | Overexpression of MYC disrupted the clock and promoted proliferation. |

In normal tissue, the circadian clock maintains homeostasis through diverse functions including control of the cell cycle, genome integrity, immunity, and metabolism. Given the numerous roles of the circadian clock in maintaining physiology, it is not surprising that the clock has been implicated in cancer initiation and progression. Indeed, a large body of evidence has linked the circadian clock to processes that become dysregulated during tumorigenesis including the cell cycle, proliferation, genome stability, stemness, metastasis, inflammation, immunity, and oncogenic signaling pathways. Analyzing over 32 different cancer types, it was found that clock genes are associated with activation or inhibition of oncogenic signaling pathways including phosphatidylinositol 3-kinase (PI3K)/AKT and RAS/mitogen-activated protein kinase (MAPK) signaling pathways (Ye et al., 2018). Knockout of Bmal1 was shown to accelerate Apc LOH in a mouse model of CRC suggesting that the clock may be involved in maintaining genome integrity (Chun, Fortin, Fellows et al., 2022). With regards to the role of the clock in the cell cycle, mutation of Cry2 in MYC-transformed fibroblasts suppressed p53 and enhanced growth (Chan et al., 2021) whereas downregulation of BMAL1 and CLOCK in human glioblastoma stem cells induced cell cycle arrest and apoptosis (Chen et al., 2020; Dong et al., 2019), demonstrating a cancer and tissue-specific effect of the clock on tumorigenesis. The circadian clock has also been shown to regulate immunity and metastasis as clock gene dysregulation is correlated with increased inflammation (Gibbs et al., 2014) and T cell exhaustion (Wu et al., 2019). Chronic jet lag promotes an immunosuppressive microenvironment, enhances stemness, and increases cancer cell metastasis (Hadadi et al., 2020) and intravasation of circulating breast tumor cells was shown to have time-of-day frequency (Diamantopoulou et al., 2022) suggesting potential clock-control of metastatic seeding. A direct link between circadian immune function and anti-tumor immunity was demonstrated by clock-dependent trafficking of DCs to the tumor draining lymph node regulating circadian function of tumor-antigen specific CD8s and melanoma volume after engraftment (Wang et al., 2022). Lastly, the circadian clock has been implicated in metabolic pathways involved in driving cellular proliferation, especially related to the crosstalk between the clock and MYC signaling (Altman et al., 2015; Chun, Fortin, Fellows et al., 2022; Shostak et al., 2016; Stokes et al., 2021). Figure created using BioRender.
As discussed in the previous section, there is a growing body of evidence suggesting a role for circadian clock disruption in various types of cancer. CRC is of particular interest as early-onset CRC is increasing at an alarming rate, faster than any other type of cancer (Augustus & Ellis, 2018; Bailey et al., 2015; Muller et al., 2021; Singh et al., 2014). Circadian disruption due to Bmal1 knockout was sufficient to drive CRC past the initiation stage (Chun, Fortin, Fellows et al., 2022; Stokes et al., 2021). The importance of multiple signaling pathways in CRC progression including Wnt, TGF-β, Notch, EGFR/MAPK and PI3K has been reviewed previously (Koveitypour et al., 2019). Here, we discuss how the circadian clock is disrupted in CRC and what molecular processes are governed by the clock that, when perturbed, result in CRC progression ( Figure 3).
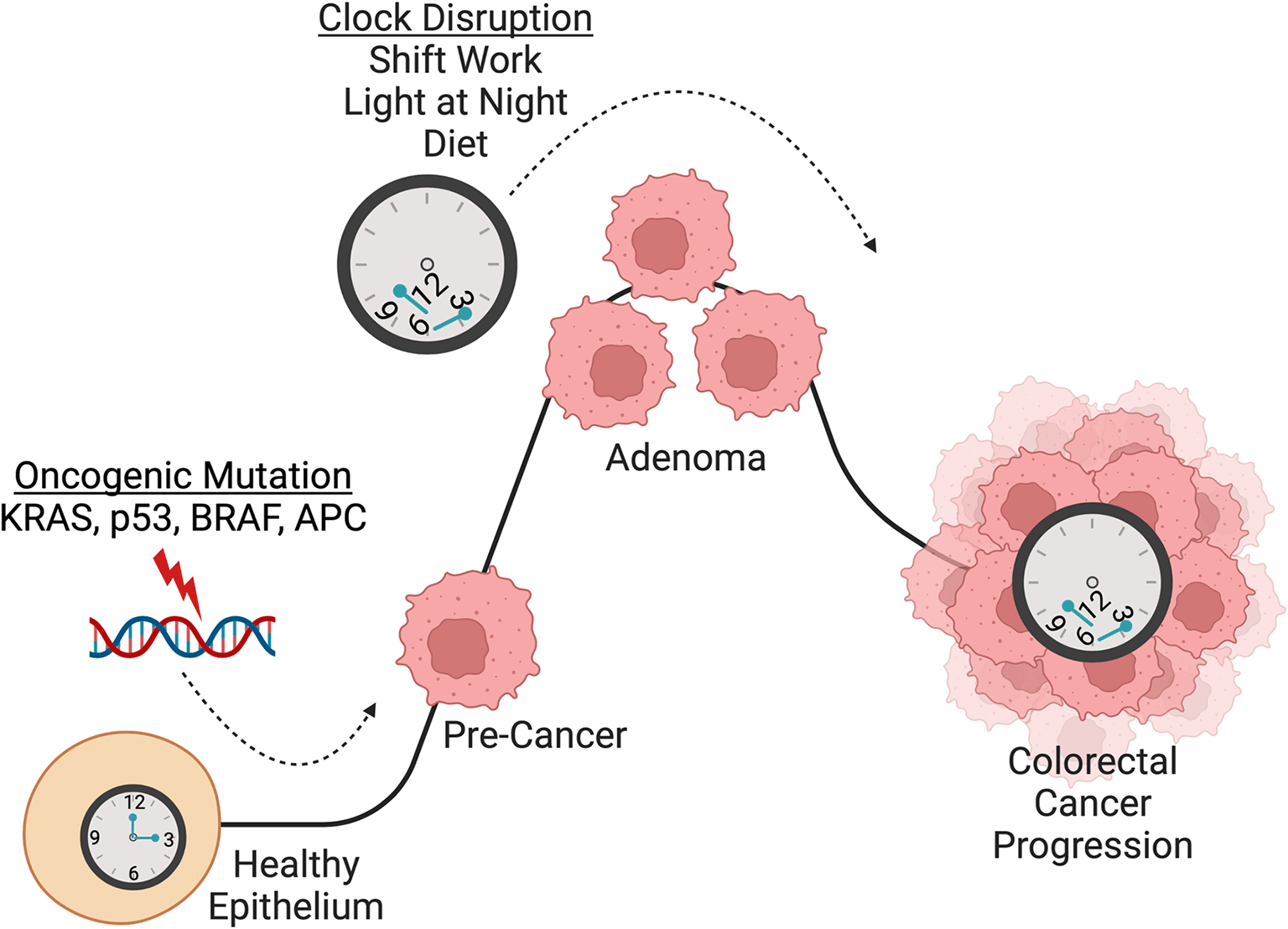
CRC has been shown to be initiated by sequential mutations in known cancer-causing genes including APC, KRAS, p53, and SMAD4 (Drost et al., 2015; Li et al., 2014). Numerous studies have found that the circadian clock is involved in CRC initiation and progression. Importantly, circadian clock disruption promotes CRC pathogenesis in multiple mouse models (Chun, Fortin, Fellows et al., 2022; Stokes et al., 2021; Wood et al., 2008). Additionally, the core clock gene CLOCK was found to be mutated in 53% of CRC that display microsatellite instability and it was shown that CLOCK binds near DNA damage related genes p21, BRCA1 and RAD50 to mediate DNA repair, apoptosis, and cell cycle arrest (Alhopuro et al., 2010). Loss of Bmal1 in Apcex1-15/+ mice and intestinal organoids accelerated Apc LOH which drove transformation (Chun, Fortin, Fellows et al., 2022). These studies implicate the circadian clock in maintenance of genome stability and demonstrate a role for circadian clock disruption in promoting colorectal carcinogenesis. An increasing number of studies have also explored the relationship between CRC and circadian clock disruption through shift work, light-at-night, and diet. Night shift work in humans has been shown to increase the risk of developing CRC (Papantoniou et al., 2017; Schernhammer et al., 2003) and chronic jet lag, through exposure to light and night, increases CRC tumor burden in mice (Chun, Fortin, Fellows et al., 2022; Stokes et al., 2021). High-fat diet also disrupts molecular circadian rhythms (Eckel-Mahan et al., 2013; Hatori et al., 2012; Kohsaka et al., 2007). Given that HFD is known to enhance tumorigenicity of intestinal progenitors (Beyaz et al., 2016; Mana et al., 2021) and exacerbate CRC (Goncalves et al., 2019), the potential link with the intestinal clock warrants further investigation. Overall, compelling evidence implicates circadian clock disruption in CRC carcinogenesis, which suggests that night shift work, light-at-night, and diet could be potential drivers of CRC progression in humans, and especially in young-onset CRC. Figure created using BioRender.
Wnt signaling is an important pathway for many processes including development, proliferation, and apoptosis (Nusse & Clevers, 2017). This is especially relevant for the intestine, a highly regenerative organ where differentiated cells are replaced every four to five days by stem cells located in the crypt base (Van Der Flier & Clevers, 2009). The Wnt pathway is upregulated when Wnt ligands bind the frizzled receptor and trigger inactivation of the destruction complex, composed of APC, GSK3-β and AXIN (Neufeld et al., 2000; Orford et al., 1997; Rubinfeld et al., 1993; Su et al., 1993). As a result, β-catenin evades proteosome-dependent degradation, and shuttles into the nucleus to co-activate TCF-LEF mediated transcription of Wnt-dependent genes (Hoverter et al., 2014; Molenaar et al., 1996).
The intestine is a highly rhythmic organ and rhythmicity is important in functions such as peristalsis, permeability and secretion of digestive enzymes (Codoñer-Franch & Gombert, 2018). The molecular clock is involved in regulating intestinal circadian rhythms as core clock genes are expressed in many intestinal cell types including stem, progenitor, tuft, enteroendocrine and enterocytes (Habowski et al., 2020). Circadian rhythms of intestinal stem cells (ISCs) are thought to be driven, at least in part, by Wnt signaling secreted from differentiated cells. ISC clocks were responsive to both Wnt and Hippo signaling in the stem cell niche (Parasram et al., 2018). Furthermore, Paneth cells were found to rhythmically secrete Wnt and many Wnt pathway components have rhythmic expression (Matsu-Ura et al., 2016; Soták et al., 2013). This is likely to be essential for proper ISC function as Wnt signaling was found to couple the clock and the cell cycle in the intestine (Matsu-Ura et al., 2016) and the coupling of clock and cell cycle is conserved across multiple species (Hong et al., 2014a; Yang et al., 2010). In the intestine, the molecular clock was found to gate cell cycle progression and was important for ISC regeneration after DSS induced damage (Karpowicz et al., 2013; Matsu-Ura et al., 2016).
The Wnt signaling pathway is highly mutated in human CRC with nearly all tumors containing inactivating mutations in APC or GSK3-β, or stabilizing mutations in CTNNB1 (β-catenin) (Kwong & Dove, 2009). Around 80% of sporadic human CRC contain a mutation in APC (Fearnhead et al., 2001). In many cases this is followed by mutation of the wild type allele, also known as loss of heterozygosity (LOH) (Fearnhead et al., 2001; Kwong & Dove, 2009). Due to its important role in the destruction complex, APC mutation results in aberrant activation of the Wnt signaling pathway (Korinek, 1997; Morin, 1997; Moser et al., 1990). APC mutations are sufficient to drive CRC initiation of adenoma growth (Cheung et al., 2010; Lamlum et al., 2000; Rowan et al., 2000; Zauber et al., 2016; Zhang & Shay, 2017). However, secondary driver mutations in key genes such as KRAS, TP53, and SMAD4 are required for progression to adenocarcinoma (Drost et al., 2015; Fearon & Vogelstein, 1990; Vogelstein et al., 1988). The circadian clock has recently been identified as a secondary driver of CRC. Bmal1 loss was found to promote Apc LOH by increasing genome instability and resulting in Wnt signaling hyperactivation in mice (Chun, Fortin, Fellows et al., 2022). Additionally, downregulation of PER2 in human colon cancer cells HCT116 and HT29 increased β-catenin levels and cell proliferation (Wood et al., 2008). Increased β-catenin in CRC cell lines also enhanced PER2 degradation by upregulating β-TrCP, an E3 ubiquitin ligase component (Yang et al., 2009). As APC is involved in regulation of cell-cell adhesion, microtubule stability, cell cycle and apoptosis (Fearnhead et al., 2001), clock disruption mediated APC LOH could perturb multiple pathways important in CRC progression.
Aside from Wnt signaling, other pathways have also been implicated in clock mediated acceleration of CRC. The Hippo pathway regulates multiple key processes including proliferation, differentiation, tissue growth, and regeneration. A cascade of serine/threonine kinases act to sequester Yes associated protein (YAP1) and transcriptional activator with PDZ binding motif (TAZ) in the cytoplasm and prevent them from activating the pro-survival TEA DNA binding (TEAD) family of transcription factors. Dysregulation results in an increase in YAP/TAZ which is associated with many human cancers, mediating increased proliferation and metastasis (Calses et al., 2019). In an Apc Min/+ mutant mouse model, YAP1 was found to be required for the progression of early initiating cells by suppressing differentiation and promoting regeneration (Gregorieff et al., 2015). The Hippo pathway may be involved in clock mediated acceleration of CRC. Yap and Tead4 increased in Apc Min/+; Bmal1 -/- mice and were associated with increased self-renewal (Stokes et al., 2021). Furthermore, Apcex1-15/+; Bmal1-/- organoids had increased expression of YAP/TAZ pathway components compared to Apcex1-15/+ organoids (Chun, Fortin, Fellows et al., 2022). In summary, clock disruption accelerates the pathogenesis of CRC, and based on data from pre-clinical studies, the circadian clock likely impinges on several important signaling pathways that regulate intestinal biology.
Misregulation of molecular clock components have frequently been identified in human CRC. Multiple studies have reported decreased BMAL1, CRY1-2 and PER1-3, and increased CLOCK, CSNK1E and TIM in tumor tissue relative to matched healthy mucosa (Hong et al., 2014b; Krugluger et al., 2007; Mazzoccoli et al., 2011, 2016; Oshima et al., 2011; Zeng et al., 2014). This was also linked to disease progression as reduced BMAL1, PER1 or PER3 expression was associated with poor overall survival (Mazzoccoli et al., 2011; Zeng et al., 2014). Additionally, clock genes were found to be mutated in cancer and therefore might be involved in pathogenesis. A large fraction of CRC patients with microsatellite instability (MSI) had a mutation in CLOCK which could decrease CLOCK expression in MSI CRC cell lines (Alhopuro et al., 2010; Mazzoccoli et al., 2011).
Pre-clinical genetic mouse models have also demonstrated that clock disruption accelerates CRC pathogenesis. In the azoxymethane and dextran sodium sulfate (AOM/DSS) model of colitis associated CRC, the circadian rhythmicity of Per1, Per2, Reverb, Dbp and Bmal1 was reduced in tumors compared to healthy colon (Soták et al., 2013). In an ApcMin/+ model of CRC, tumors exhibited reduced overall expression of key clock components Rev-Erbα, Bmal1 and Per2, with complete loss of Per2 rhythm (Stokes et al., 2021; Yang et al., 2009). Furthermore, clock disruption was found to increase tumor burden when Per2 or Bmal1 were deleted in ApcMin/+ mice (Stokes et al., 2021; Wood et al., 2008). In a novel mouse model, where exons 1 to 15 in Apc were deleted in one allele (Apcex1-15/+), Bmal1 knockout increased tumor incidence, enlarged polyps and decreased survival (Chun, Fortin, Fellows et al., 2022). Additionally, environmental circadian disruption through use of a light shift paradigm or constant light increased both polyp formation and size of tumors in Apcex1-15/+ and Apcmin/+ mice (Chun, Fortin, Fellows et al., 2022; Stokes et al., 2021). Together these results suggest that circadian disruption can play a key role in driving pathogenesis of CRC.
In modern society, the necessity of night shift work and the presence of artificial light at night warrants a better understanding of the impact of circadian disruption on health and disease. Above, the literature defining circadian clock function in critical cellular processes and the role of circadian clock disruption in various cancer types was summarized. In this section, emerging ideas for how the circadian clock can be leveraged to both prevent and treat cancer are highlighted. For additional information on this topic, a more extensive review of chronotherapeutic approaches has recently been published (Sulli et al., 2018a).
Promoting robust circadian rhythms through consistent sleep and feeding behavior is an important regulator of physiological health. However, night shift workers are faced with irregular activity-rest and feeding-fasting rhythms as well as artificial light at night exposure, all of which are known to disrupt the circadian clock. Key literature was reviewed above that aimed to define the correlation between night shift work and cancer prevalence. Although this body of literature requires more comprehensive studies to draw definitive conclusions, the importance of proper alignment of circadian rhythms has emerged as a key theme. Numerous studies cite a significant increase in cancer risk after long-term night shift work, typically 15–20 years (Åkerstedt et al., 2015; Hansen & Lassen, 2012; Wegrzyn et al., 2017). Risk was also seen to increase with duration of exposure (Davis et al., 2001; Hansen & Lassen, 2012; Hansen & Stevens, 2012; Lie et al., 2011). For example, in nurses who worked night shift for over five years, the risk of developing breast cancer increased from an odds ratio of 1.4 to 1.8 with increasing consecutive night shifts (Lie et al., 2011). In order to reduce the risk of cancer in night shift workers, the consecutive duration of night shifts may need to be limited as well as the cumulative exposure to night shift work.
Circadian misalignment, defined as a disruption or misalignment between an individual’s internal circadian clock and the external cues such as light-dark and feeding-fasting cycles, is an unavoidable consequence of night shift work. However, recent research has recommended lifestyle interventions as a means of combating these effects. Night shift workers often disrupt their feeding-fasting patterns which has been shown to disrupt glucose metabolism (Spiegel et al., 1999). Night shift work has also been significantly associated with metabolic syndrome (Wang et al., 2014), which increases the risk for developing various types of cancer (Esposito et al., 2012). Therefore, increasing metabolic health through lifestyle intervention may combat the increased risk of cancer. One such intervention is time-restricted eating (TRE), which involves limiting the eating window to 6–12 hours per day (Manoogian et al., 2022). In a study with prediabetic men, limiting the feeding window to six hours for five weeks improved insulin sensitivity, blood pressure, oxidative stress, and appetite (Sutton et al., 2018). A recent study found that a 10-hour feeding window in 24-hour shift workers is a feasible intervention to reduce weight, improve cardiometabolic health, sleep quality, and mood (Manoogian et al., 2022).
It has been well established that night shift is associated with circadian misalignment, however, the general population is increasingly at risk of circadian misalignment through inconsistent eating patterns and artificial light-at-night exposure. Therefore, it may be beneficial to update cancer screening measures (Patel et al., 2022; Wolf et al., 2018). As the percentage of early-onset cancer increases, the screening age should decrease accordingly for early detection and prevention measures. Promoting robust circadian rhythms through consistent sleep, feeding, regulation of night shift work, and lifestyle interventions such as TRE may help improve parameters that impinge on human health and could offset the impacts of circadian misalignment on specific cancer types.
Cancer chronotherapy refers to the timing of an anticancer drug to increase efficacy and decrease toxicity. This approach is based on the rationale that the drug will be better tolerated at certain times of day based on the mechanism of action of the drug. A recent comprehensive review of chronomodulated chemotherapy has recently been reported (Printezi et al., 2022). In this systematic review, 11 of 18 studies found that chronomodulated chemotherapy significantly decreased toxicity while maintaining efficacy. More specifically, chronomodulated chemotherapy reduced side effects including nausea, vomiting, mucositis and leukopenia for nasopharyngeal carcinoma (Gou et al., 2018; Zhang et al., 2018), breast (Coudert et al., 2008), colorectal (Lévi et al., 1994, 1997) and endometrial cancer (Gallion et al., 2003). Reducing side effects is a critical aspect of patient care as it improves quality of life and often allows for higher or more frequent doses. Although chronomodulated chemotherapy does appear to reduce side effects, it remains elusive whether chronotherapy improves drug efficacy or prognosis. For example, only three studies report higher response rate and longer survival in chronotherapy treated groups (Gou et al., 2018; Lévi et al., 1994, 1997). However, two of the three of these studies report higher dose intensity in the chronotherapy treated group because of the reduced side effects. Therefore, it is unclear whether improved response is due to increased dose or a direct result of chronotherapy. A meta-analysis on factors impacting drug timing effects found that study size and whether or not the study was publicly registered as a clinical trial affected the reported efficacy of chronomedicine (Ruben et al., 2021), suggesting that further studies are needed. In summary, optimal timing of anticancer drugs appears to reduce toxicity but more mechanistic studies are needed to determine the clinical relevance of anticancer chronotherapeutic approaches on drug efficacy.
The circadian clock is an evolutionarily conserved internal timekeeping system that maintains homeostasis within the body. In this review, we discussed the connection between the circadian clock and critical biological processes including cell cycle control, DNA damage response, DNA repair, and immunity. Disruption of these processes are known hallmarks of cancer (Hanahan, 2022; Hanahan & Weinberg, 2000, 2011), and we highlight the links between circadian clock disruption and cancer through clinical, epidemiological, and pre-clinical molecular studies. Though progress has been made to deconvolute the role of the circadian clock in cancer, this review highlights the divergent evidence linking circadian clock disruption with tumorigenesis. For clinical and epidemiological studies, these differing conclusions may be due to self-reporting, confounding factors, and non-standardized definitions of night shift work. For molecular and mechanistic studies, clock-controlled rhythmic expression is known to be tissue-specific, suggesting that the impact of circadian clock disruption would also be tissue specific. This makes drawing a simplified conclusion regarding the role of clock disruption on tumorigenesis difficult. Future studies are needed to systemically explore these tissue-specific differences and determine the role of clock disruption in each organ independently. These more comprehensive studies will yield a foundational understanding by which the circadian clock can be leveraged for cancer prevention and chronomedicine-based approaches.
Finally, with the alarming rise in the rate of early-onset cancers and the necessity of night shift work in modern society, it is imperative to address the concern of circadian clock disruption in a growing population of individuals afflicted by circadian misalignment. In this review, we highlighted how individuals can promote healthy circadian rhythms by limiting the exposure to night shift work, lifestyle interventions such as TRE, and updated cancer screening. This list is not comprehensive and additional molecular studies are needed to guide our understanding of intervention approaches that can offset circadian clock disruption. Overall, the circadian clock presents a unique and underexplored connection between health and disease which has the potential for therapeutic value in cancer treatment.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Circadian rhythms, stem cells
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Yes
References
1. Moreno-Smith M, Milazzo G, Tao L, Fekry B, et al.: Restoration of the molecular clock is tumor suppressive in neuroblastoma.Nat Commun. 2021; 12 (1): 4006 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Circadian rhythms
Is the topic of the review discussed comprehensively in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Is the review written in accessible language?
Yes
Are the conclusions drawn appropriate in the context of the current research literature?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Circadian rhythms, stem cells
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 16 May 23 |
read | |
|
Version 1 01 Feb 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)