Keywords
Ethanolic Extract Ocimum sanctum Lin;, Breast Cancer; RNS; Insilico molecular docking; An-ti-proliferative; Apoptosis; Caspase 3; Caspase 9
This article is included in the Oncology gateway.
This article is included in the Cell & Molecular Biology gateway.
Ethanolic Extract Ocimum sanctum Lin;, Breast Cancer; RNS; Insilico molecular docking; An-ti-proliferative; Apoptosis; Caspase 3; Caspase 9
Cancer is becoming more common globally. Breast cancer is a serious illness and the leading cause of death, accounting for 23% of all cancers and 14% of cancer-related deaths in women worldwide.1 Increasing age, reproductive, and genetic factors are known risk factors for breast.2 Breast cancer is a common female malignancy, affecting 2.1 million women annually.3 Surgery, radiotherapy, chemotherapy, endocrine therapy, and targeted therapy are breast cancer treatment strategies. Chemotherapy plays an important role in breast cancer treatment.4 It is widely used in combination with surgery and radiation therapy, although the efficacy of this treatment is still the leading cause of death.5 Chemotherapy, as a cancer treatment, uses cytotoxic agents to kill cancer cells. This agent destroys tumor tissue and cannot distinguish normal cells from cancer, moreover, the method is not effective if cancer already undergoes metastases. Therefore, new strategies to effectively control cancer growth are urgently needed.6 While modern and conventional medicine has been used as the main medicine for cancer treatment, traditional herbal medicine has been commonly used as a complementary and alternative strategy in several countries.7
Plants have long been known for their therapeutic properties. Herbal products can be trusted for cancer treatment because of their low toxicity. Most women with breast cancer easily accept herbal medicines because of their availability, affordable prices, ease of implementation, and acceptance to control cancer cell growth.8 In these relations, it is important to mention that Ocimum sanctum Linn. (OS) is an herbal plant native to Asian countries that has several beneficial biological activities,9 especially anti-inflammatory10 and antioxidant activity.11 However, relatively few studies have investigated the biological activity underlying the cytotoxic effect of EEOS treatment in altering the survival of lung adenocarcinoma A549 cells through the synergistic induction of apoptosis signaling via the intrinsic mitochondrial pathway,12 the ethanolic extract of OS inhibited A549 cell angiogenesis by how to reduce the expression of αvβ3 integrins, MMP-2, and MMP-9.13 To provide scientific justification for the use of herbal medicines, it is necessary to record and publish preclinical evidence and clinical-based research. Therefore, this study was designed to analyze the ability of the ethanolic extract of O. sanctum Linn leaves as an anticancer agent in breast cancer cells, mainly to prove the ability to inhibit the proliferation of cancer cells.
The leaves of Ocimum sanctum Linn simplisia were obtained from center of agrotechnology, Universitas Gadjah Mada and the species identified at the department of biology, Universitas Gadjah Mada (Yogyakarta, Indonesia). The ethanolic extract was obtained by maceration technique.12 A total of 4000 ml of 96% ethanol (Merck, Darmstadt, Germany) was added to 300 grams simplicia Ocimum sanctum Linn. The filtration results concentrated using a vacuum rotary evaporator (Heidolph, Schwabach, Germany), and 8.82% w/w of ethanol extract of Ocimum sanctum Linn was obtained in the form of a paste.
MCF-7 and T47D cells were cultivated in T25 flasks with Dulbecco's Modified Eagle Medium (DMEM) (Gibco, Langenselbold, Germany) supplemented with Fetal Bovine Serum (FBS) (Capricorn, Ebsdorfergrund, Germany) 10%, Penicillin-Streptomycin (Capricorn, Ebsdorfergrund, Germany) 2%, amphotericin B 0.5%, and Non-Essential Amino Acids (NEAA) 1%. The T25 flask was then stored in an incubator at 37°C and 5% CO2. T47D cells were cultured in T25 flasks with Roswell Park Memorial Institute (RPMI) 1640 media (Gibco, Langenselbold, Germany) supplemented with Fetal Bovine Serum (Capricorn, Ebsdorfergrund, Germany) 10%, penicillin-streptomycin (Capricorn, Ebsdorfergrund, Germany) 2%, and 0.5% amphotericin-B, and stored in an incubator at 37°C and 5% CO2.
Cytotoxicity ability of EEOS examined by MTT assay (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide). 80% confluence cell of MCF-7 and T47D breast cells were grown on a 96-well culture test plate and incubated at 37°C and 5% CO2 for 24 hours. Cell cultures were rinsed with Dulbecco's Phosphate Buffer Saline (DPBS) (Capricorn, Ebsdorfergrund, Germany) prior to treatment. Cells were divided into untreated (NT), cisplatin (Kalbe, Jakarta, Indonesia) 35 μg/ml (MCF-7) and 50 μg/ml (T47D cells) groups as positive controls with commercial drugs for the treatment of breast cancer, and EEOS with graded concentrations of 50, 100, 150, 200, and 250 μg/ml (MCF-7) and 50, 75, 150, 225, and 300 μg/ml (T47D cells). The analysis was run as triplicate. The treated cells were incubated at 37°C and 5% CO2 for 24 hours. The media was removed and rinsed with DPBS, then 0.5 mg/ml MTT reagent was added to 100 l/well. Cells were incubated for 4 hours and then given Dimethyl sulfoxide (Sigma-Aldrich, Munich, Germany) 100 μl/well to stop the formation of formazan crystals. The optical density value was read using a microplate reader (Tecan, Zurich, Switzerland) with a wavelength of 595 nm. The results of the absorbance optical density value are calculated using the formula:
The ability of EEOS to inhibit the adhesion of breast cancer cells was examined by the adhesion test using the CCK-8 test (Cell Counting Kit-8) (Abbkine cat: KTC011001-3, California, USA) with Water Soluble Tetrazolium (WST)-8 reagent. MCF-7 and T47D breast cancer cells were grown on 96 well culture test plates and incubated at 37°C and 5% CO2 for 24 h. Cells were then treated by EEOS. After being treated and incubated for 24 h, the media was removed and rinsed with DPBS, then 100 l of 10% WST-8 reagent was added. Cells were incubated for 1 h. The optical density value was read using a microplate reader (Tecan, Zurich, Switzerland) with a wavelength of 450 nm. The results of the absorbance optical density value are calculated using the formula:
Confluent MCF-7 and T47D breast cancer cells were grown on 6-well culture test plates and then incubated at 37°C and 5% CO2 for 24 h. Cells were washed with DPBS and then treated with EEOS after incubated for 24 hours, rinsed the cultivation with DPBS and lysed using 1000 μl of RIPA lysis buffer. The adhering cells were removed with the aid of a cell scraper and then transferred to micro-tubes and centrifuged at 6500 rpm for 10 min at 4°C. The resulting supernatant was taken for the Griess test.
The MCF-7 and T47D cell lysates supernatants (100 μl) and standard solutions were transferred to 96 wells assay plate culture. Add 0.1% N-1-naphtyletthylenediamine dihydrochloride (NEDD) and 2% sulfanilamide in 5% HCl 50 l each to each well simultaneously. The absorbance value was read by a microplate reader with a wavelength (λ) of 540 nm. The average value of the absorbance was compared with the standard value of nitride oxide (NO) to obtain the NO concentration.
Interventionary studies involving animals or humans, and other studies that require ethical approval, must list the authority that provided approval and the corresponding ethical approval code.
Confluent MCF-7 and T47D breast cancer cells were grown on a 24-well culture test plate, covered with a sterile coverslip and allowed for 30 minutes, then added 200 μl of media and incubated at 37°C and 5% CO2. for 24 h. Cells were then treated with EEOS and carried out in duplicate. Cells were rinsed with DPBS and fixed using 250 μl of 2.5% glutaraldehyde, cells were incubated for 2 hours at 4°C. The slides were then dehydrated using a series of graded ethanol solutions (20, 30, 40, 50, 60, 70, and 100%). The specimens were vacuum dried (25°C, 4 Pa; Buehler 1000 Vacuum System, Stuttgart, Germany) and coated with platinum (JEOL JEC-3000FC, Tokyo, Japan), then examined under an SEM (JEOL-JSM6510LA, Tokyo, Japan) using a 15-kV acceleration voltage.
The four bioactive compounds of the EEOS were identified as apigenin (CID_5280443),43 caffeic acid (CID_689043),44 and Gallic acid (CID_370),45 dan rosmarinic acid (CID_5281792),46 downloaded from the PubChem database (see Data availability).
The target proteins used in this in silico study were caspase-9 (PDB ID:1jxq)47 and caspase-3 (PDB ID: 1nms),48 which are downloaded from the structural information PDB.
Bioactive compounds (apigenin, gallic acid, caffeic acid, and rosmarinic acid) and target proteins (caspase-9 and caspase-3) were prepared sequentially using the Discovery Studio v. 19.0.0 program and the PyRX 0.8 program. The proteins and bioactive compounds were interacted with the HEX 8.0.0 program and visualized with the Discovery Studio Visualizer v. 19.0.0.
MCF-7 and T47D cells were cultured on well plates and treated for 24 hours with different concentrations of EEOS. MCF-7 cells are treated with EEOS in 50, 100, 150, 200, and 250 μg/ml concentrations. In another hand, T47D cells are treated with 75, 150, 225, 300, and 375 μg/ml EEOS. Cisplatin is given as positive control as a comparison to commercial drugs. EEOS induced cell death and inhibit cell attachment in MCF-7 cell dose-dependent manner (Figures 1A, 2A) the optimal concentration of EEOS, 100 μg/ml, showed a little amount of attaching cell in comparison with other concentrations (Figure 1). Furthermore, EEOS also induced cell death and inhibit cell attachment, but non-significant in 75 and 150 μg/ml concentration of EEOS (Figures 1B, 2B). The optimum concentration EEOS to inhibit the growth of T47D cells is 375 μg/ml and showed enormous cell detachment with others.
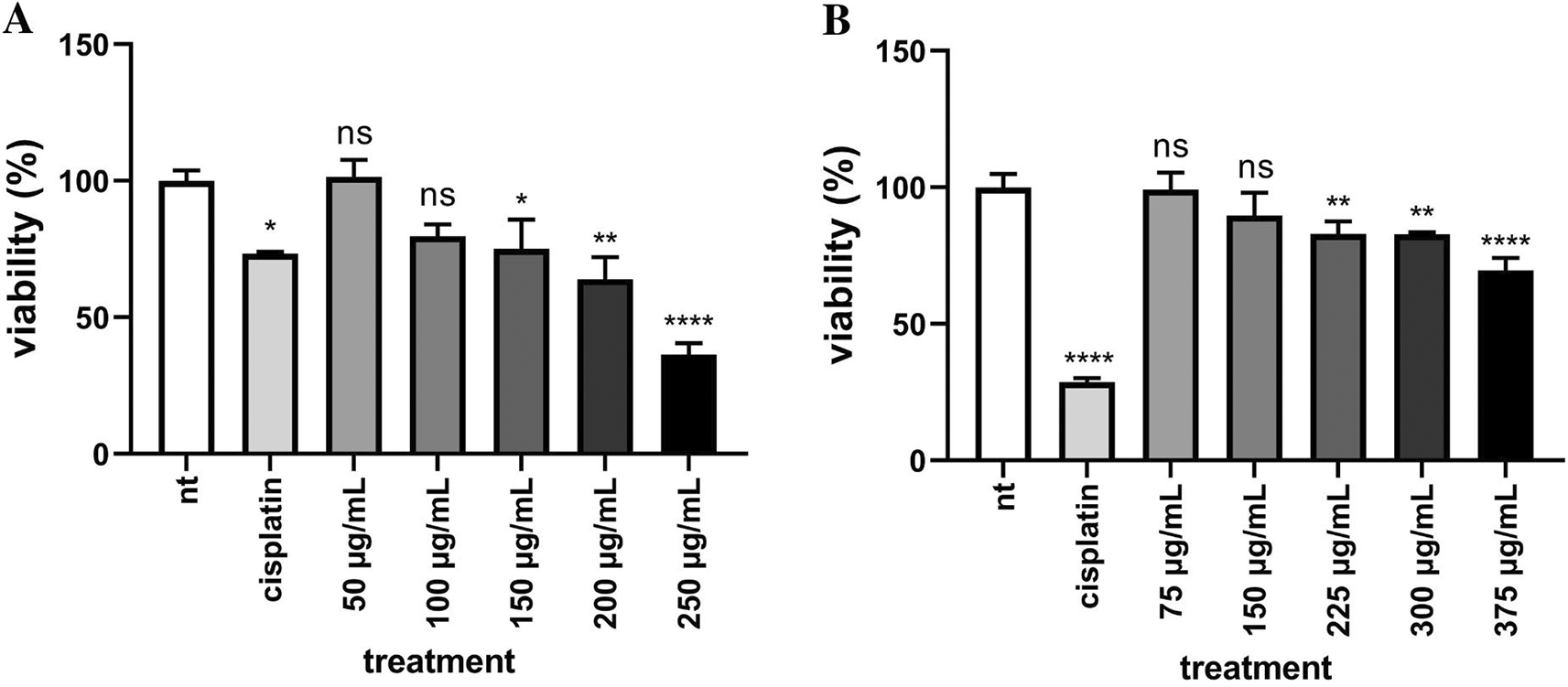
(EEOS) against MCF-7 (A) and T47D (B) breast cancer cells. MCF-7 breast cancer cells (A) were cultivated in the presence of cisplatin 35 μg/ml as the commercial drug comparison, and EEOS at concentrations of 50, 100, 150, 200, dan 250 μg/ml. T47D breast cancer cells (B) were cultivated in the presence of cisplatin 50 μg/ml as the commercial drug comparison, and EEOS at concentrations of 75, 150, 225, 300, and 375 μg/ml. After 24 h, EEOS’s inhibitory effect was visualized by an MTT reagent at a wavelength of 595 nm (NT: Non-treated; **significant p<0.0062; ****significant p<0.0001; n.s.=Not significant).
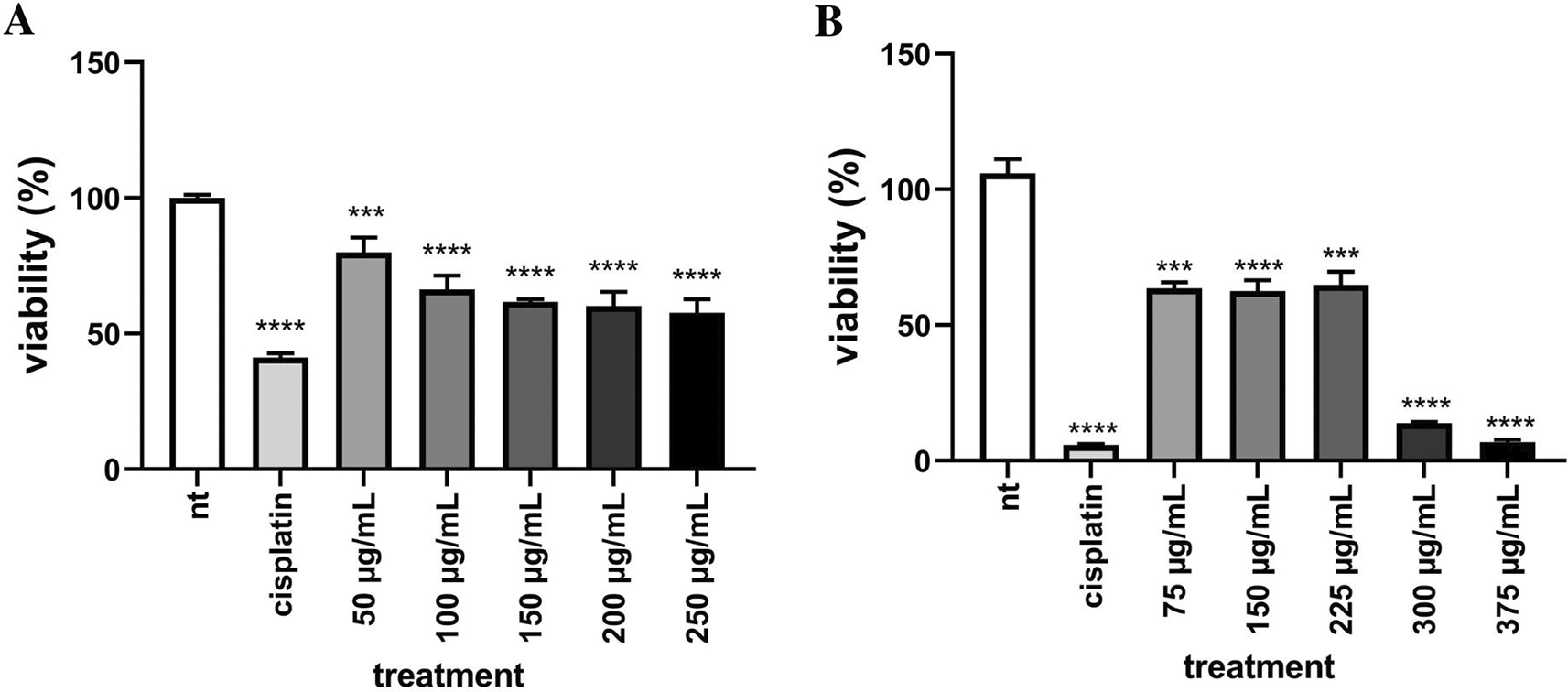
(EEOS) against MCF-7 (A) and T47D (B) breast cancer cells. MCF-7 breast cancer cells (A) were cultivated in the presence of cisplatin 35 μg/ml as the commercial drug comparison, and EEOS at concentrations of 50, 100, 150, 200, dan 250 μg/ml. T47D breast cancer cells (B) were cultivated in the presence of cisplatin 50 μg/ml as the commercial drug comparison, and EEOS at concentrations of 75, 150, 225, 300, and 375 μg/ml. After 24 h, EEOS’s inhibitory effect was visualized by an MTT reagent at a wavelength of 595 nm (NT:Non-treated; **significant p<0.0062; ****significant p<0.0001; n.s.=Not significant).
In addition, SEM was performed to visualize the morphological surface of MCF-7 (Figure 3) and T47D (Figure 4) breast cancer cells. In the administration of EEOS with gradual concentrations on MCF-7 cells: 50 μg/ml EEOS (Figure 3C), 100 μg/ml EEOS (Figure 3D), 150 μg/ml EEOS (Figure 3E), 200 μg/ml EEOS (Figure 3F), and 250 μg/ml (Figure 3G), a protrusion appeared on the cell surface followed by the formation of holes on the cell surface, where more holes appeared as the concentration of EEOS was increased. In addition, the experiment was also performed In T47D cells treated in the presence of EEOS at gradual concentrations. Several concentrations of EEOS are 50 μg/ml EEOS (Figure 4C), 75 μg/ml EEOS (Figure 4D), 150 μg/ml EEOS (Figure 4E), 225 μg/ml EEOS (Figure 4F), and 300 μg/ml EEOS (Figure 4G). In the treatment of EEOS, the T47D showed quite clear changes; the cells looked more gritty, with holes and blebbing on the surface of the cells; at the highest concentration (300 μg/ml), the cells appeared flattened and more holes (Figure 4). Morphological changes in both cell lines, the appearance of roughness, holes, or blebbing, are markers that MCF-7 and T47D cells undergo apoptosis. These results are also consistent with the surface structure of MCF-7 cells (Figure 3B) and T47D (Figure 4B) with the administration of cisplatin as one of the commercial drugs used to treat breast cancer today.
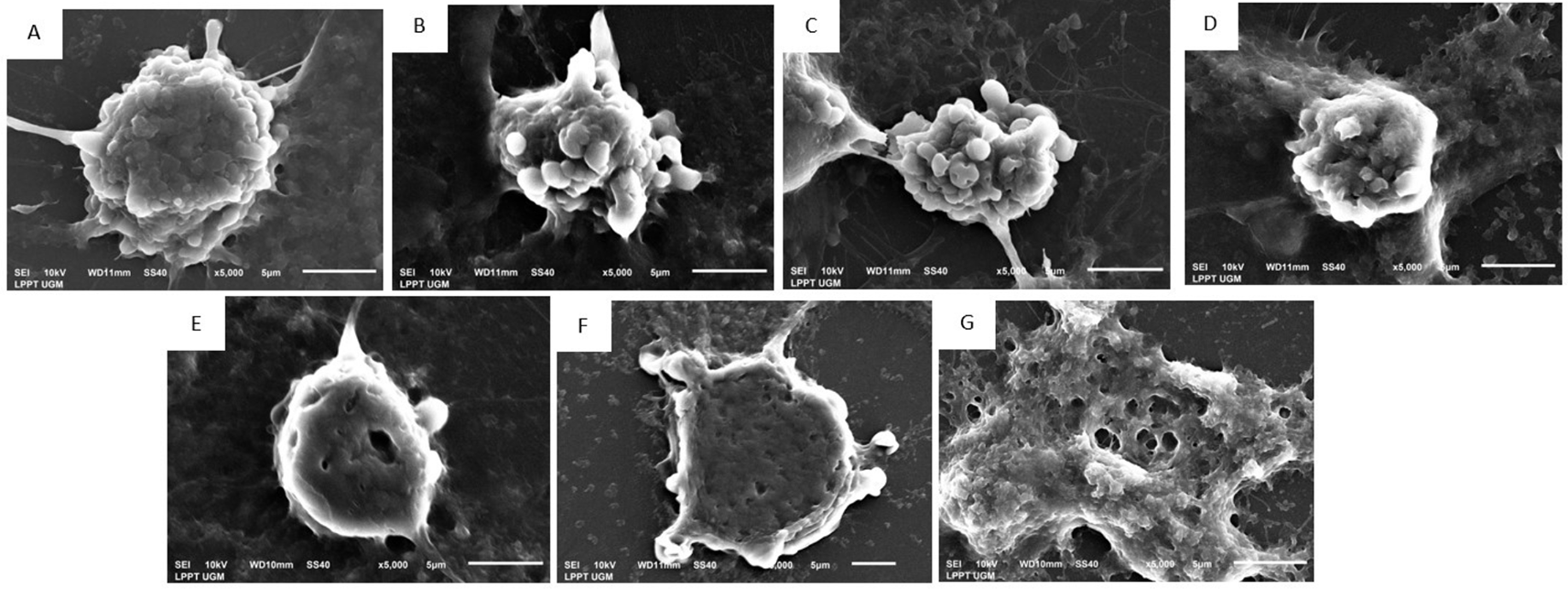
(EEOS) A) Non treated cells, in the morphologycal analysis of the cells show no cells membrane blebing and hole on the cell surface, (B) MCF-7 cells in the presence of Cisplatin 35 μg/ml, on the surface of the cells, the cells blebing are appear, in addittion the same characteristic of necrotic or apoptotic cells also found in MCF-7 in the presence 50 μg/ml EEOS (C), and furthermore in the treatment of 100 μg/ml EEOS (D), 150 μg/ml EEOS (E), treatment with 200 μg/ml EEOS (F), and treatment with 250 μg/ml (G) beside the blebbing, the cells have more hole or pit on the surface which indicate the cells undergo to apoptosis.
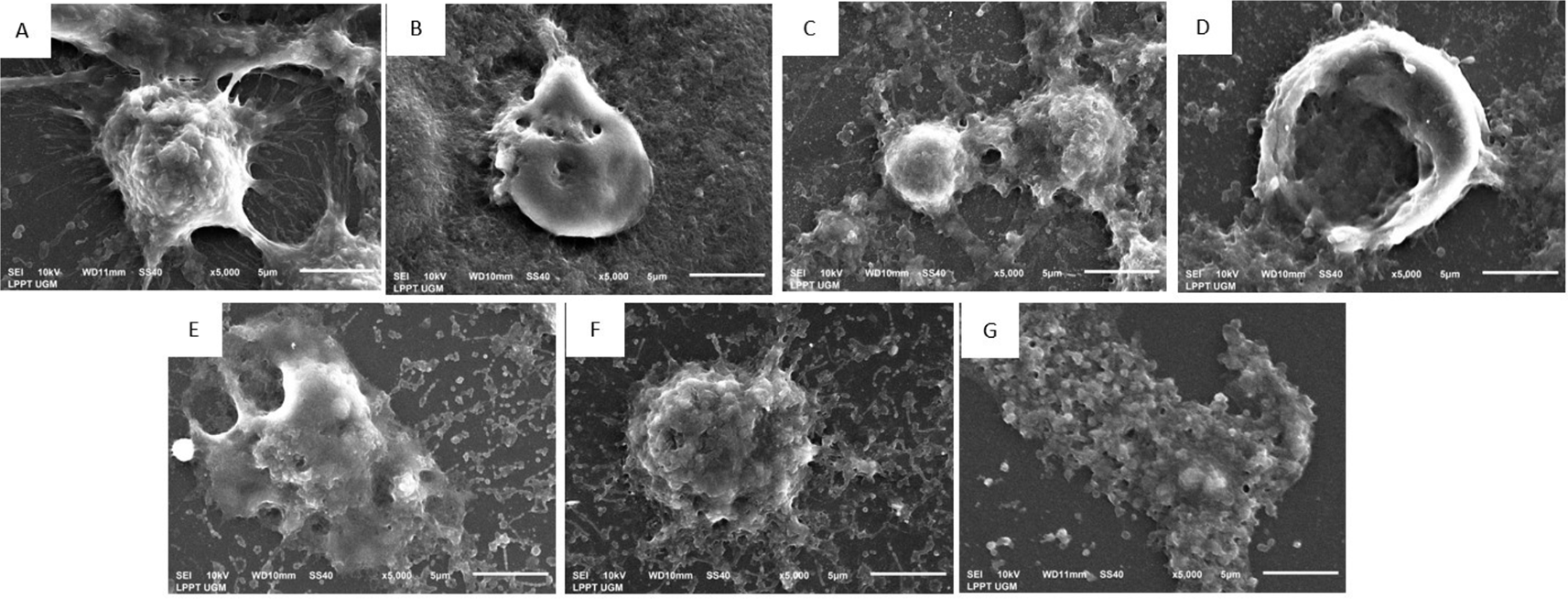
(EEOS) A) Non treated cells, in the morphologycal analysis of the cells show no cells membrane blebing and hole on the cell surface, (B) T47D cells in the presence of Cisplatin 35 μg/ml, on the surface of the cells, the cells hole are appear, in addittion the same characteristic of necrotic or apoptotic cells also found in T47D in the presence 50 μg/ml EEOS there is some blebbing and hole in the surface of the cells(C), meanwhile on 75 μg/ml EEOS the holes more appear on the surface(D), furthermore in the treatment of 150 μg/ml EEOS (E), 225 μg/ml EEOS (F), and treatment with 300 μg/ml (G) the cells have more hole or pit as well as blebbing on the surface. In addition in the presence of 300 μg/ml (G) the cells describe more flattened with blebbing and hole in compare to non trated cells (A). The changes of cells morphology indicate the cells undergo to apoptosis.
To demonstrate the ability of EEOS to induce an apoptotic effect on MCF-7 and T47D breast cancer cells we performed Griess assay to measure the concentration of Nitric Oxide (NO) as a part of Reactive Nitrogen Species (RNS). Indeed, this experiment showed that concentration of NO in MCF-7 and T4TD breast cancer cells were increased in line with increase in EEOS concentration (Figure 5).
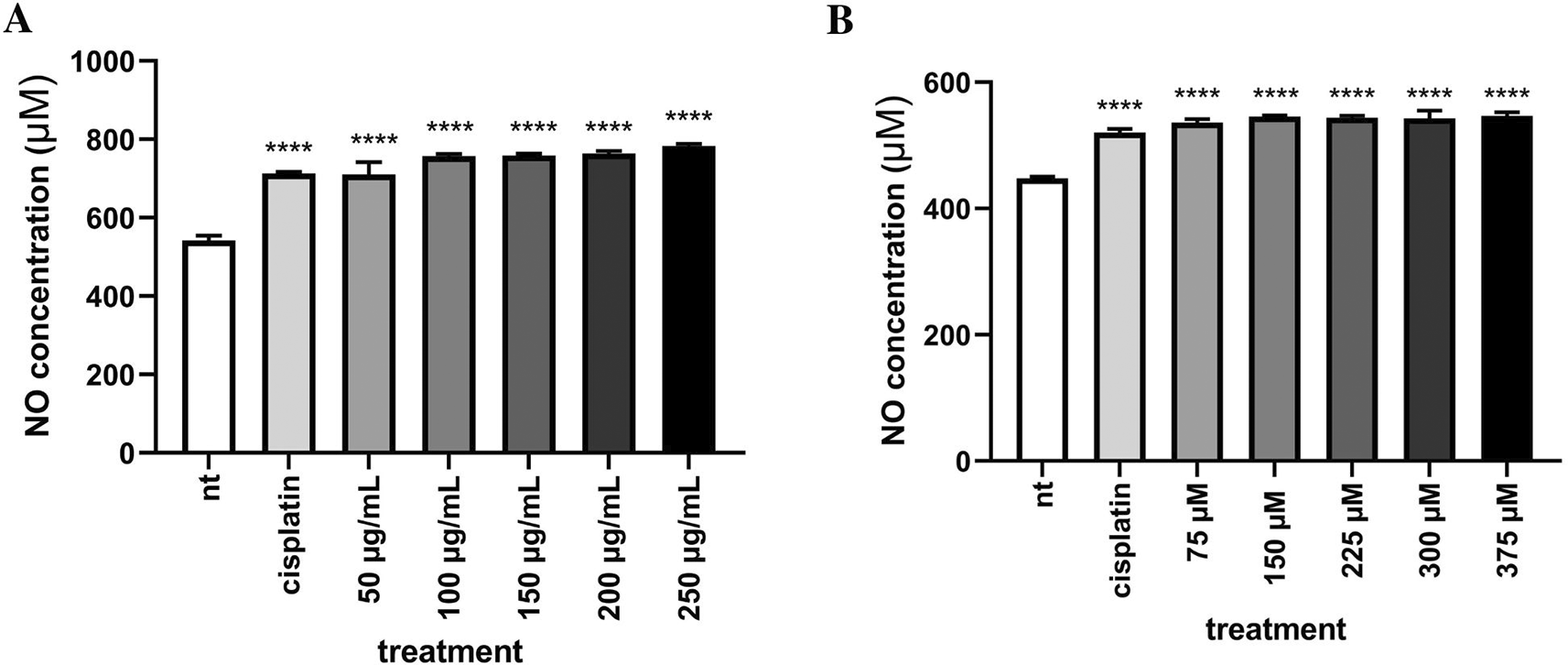
MCF-7 breast cancer cells (A) were cultivated in the presence of cisplatin 35 μg/ml as the commercial drug comparison, and EEOS at concentrations of 50, 100, 150, 200, dan 250 μg/ml. T47D breast cancer cells (B) were cultivated in the presence of cisplatin 50 μg/ml as the commercial drug comparison, and EEOS at concentrations of 75, 150, 225, 300, and 375 μg/ml. After 24 h, EEOS’s inhibitory effect was visualized by an MTT reagent at a wavelength of 595 nm (NT:Non-treated; **significant p<0.0062; ****significant p<0.0001; n.s.=Not significant).
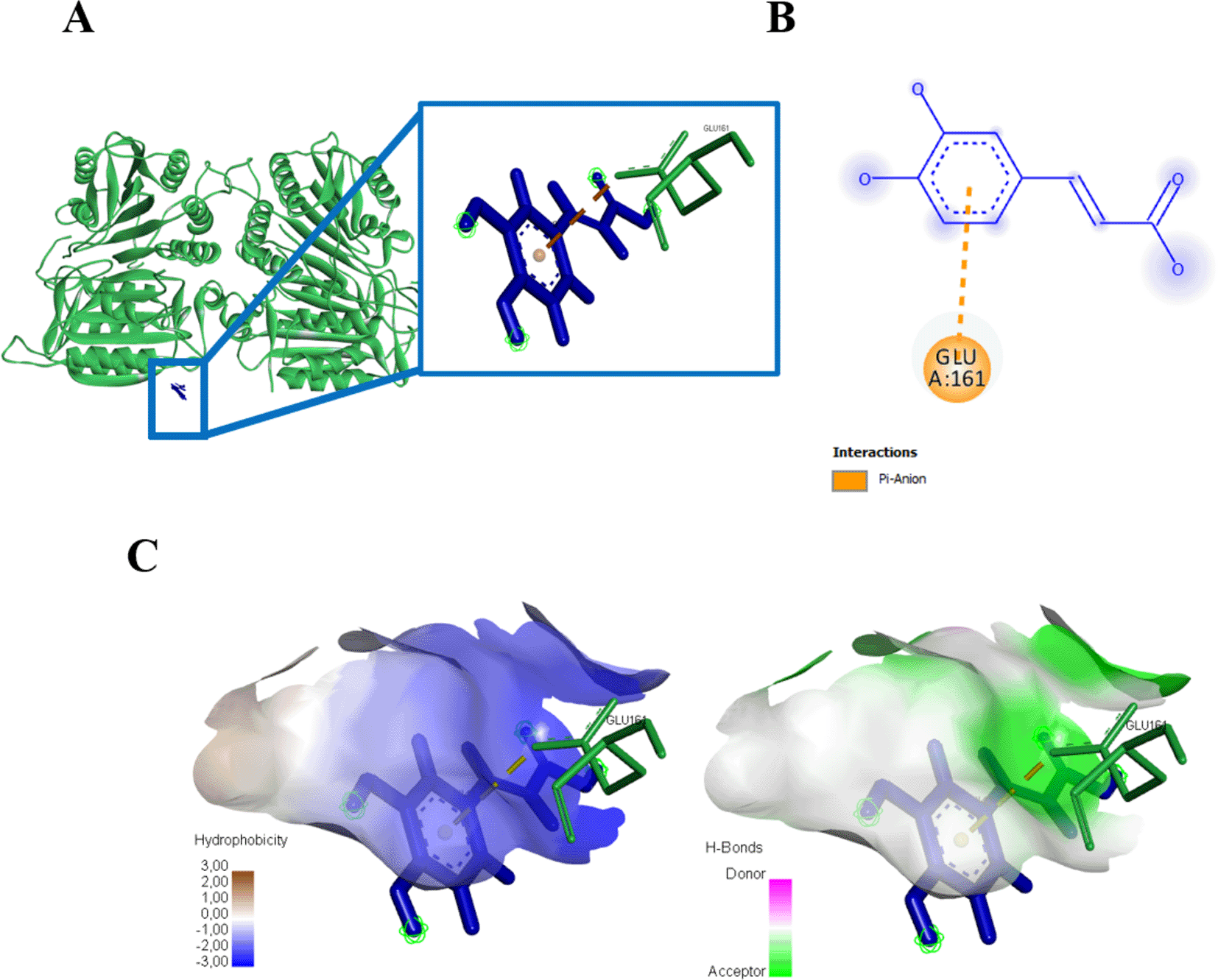
Three amino acid residues of protein caspase 3 bound to gallic acid in THR166, GLU123, and GLU167 with an energy of -167.9 kJ/mol (Figure 6, Table 1). Gallic acid compounds showed different interaction results when docked with caspase 9, two amino acid residues (SER194, GLU161) were found that interacted with and bond energy of -101.9 kJ/mol (Figure 7, Table 1).
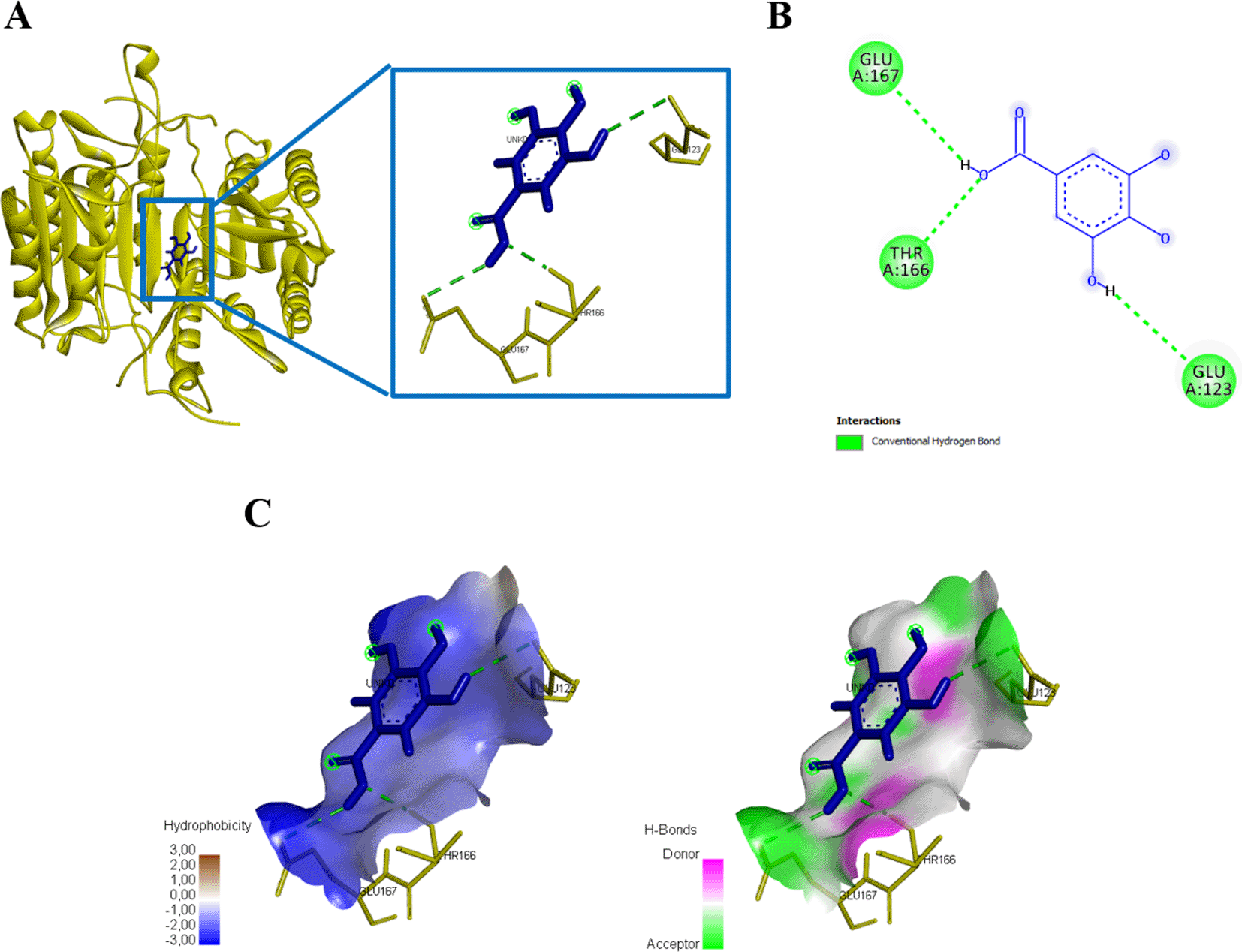
(A) Three-dimensional structural interaction between the ligand Gallic Acid (blue) and caspase 3 (yellow). This interaction between Gallic Acid on the active site of caspase 3, called THR166, GLU123, GLU167, which can be seen in the two-dimensional (B) and three-dimensional structures (C).
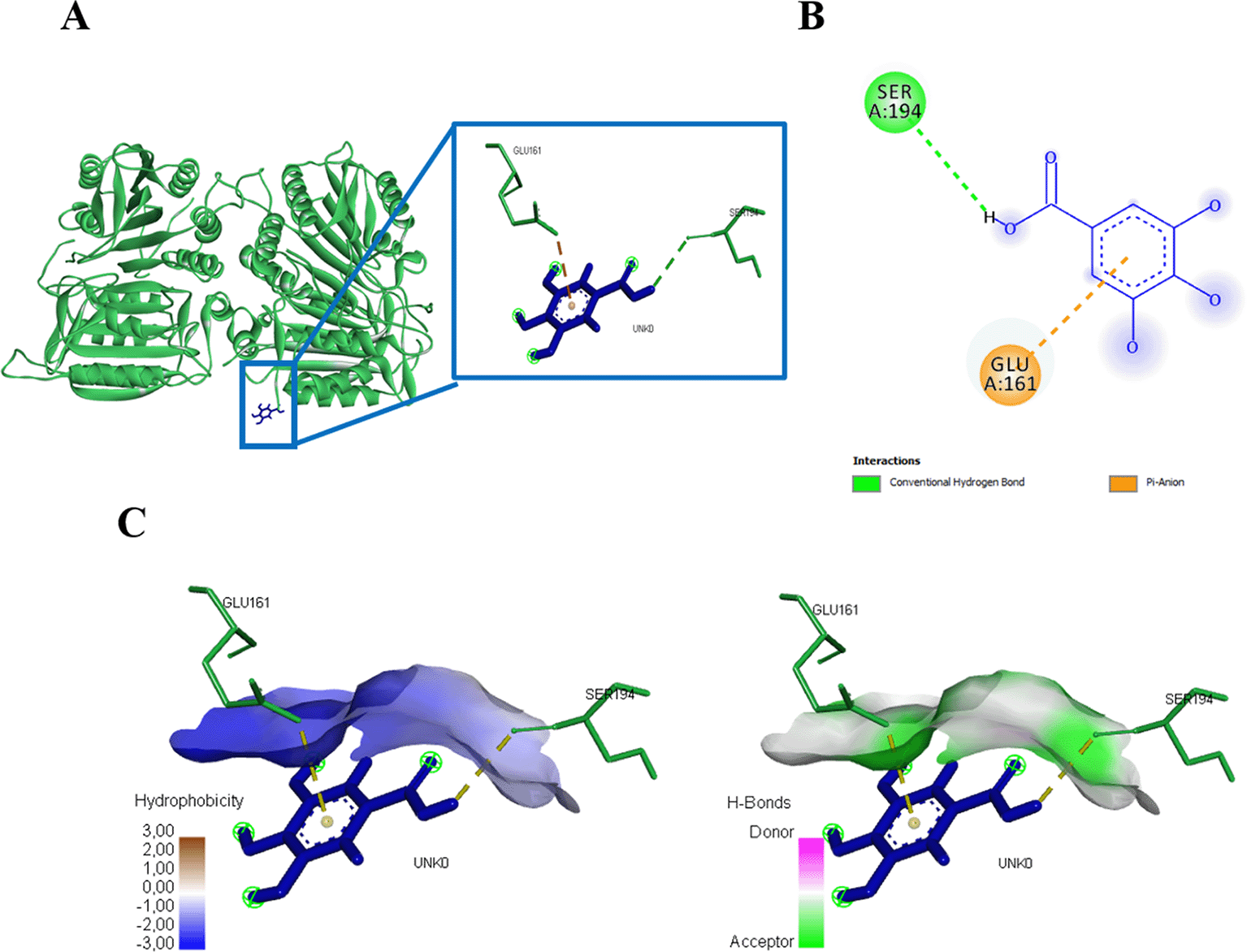
(A) Three-dimensional structural interaction between the ligand Gallic Acid (blue) and caspase 9 (green). This interaction between Gallic Acid on the active site of caspase 9, called, SER194, GLU161, which can be seen in the two-dimensional (B) and three-dimensional structures (C).
Caffeic acid compounds and protein caspase 3 binds to amino acid residues ASN35, ARG241, and THR245, this interaction forms affinity energy of -214.4kJ/mol (Figure 8, Table 2). Caffeic acid compounds that interacted with protein caspase 9 showed activity with total energy -117.0kJ/mol, the interaction found that there was one amino acid residue that interacted with caffeic acid, namely GLU161 (Figure 9, Table 2).
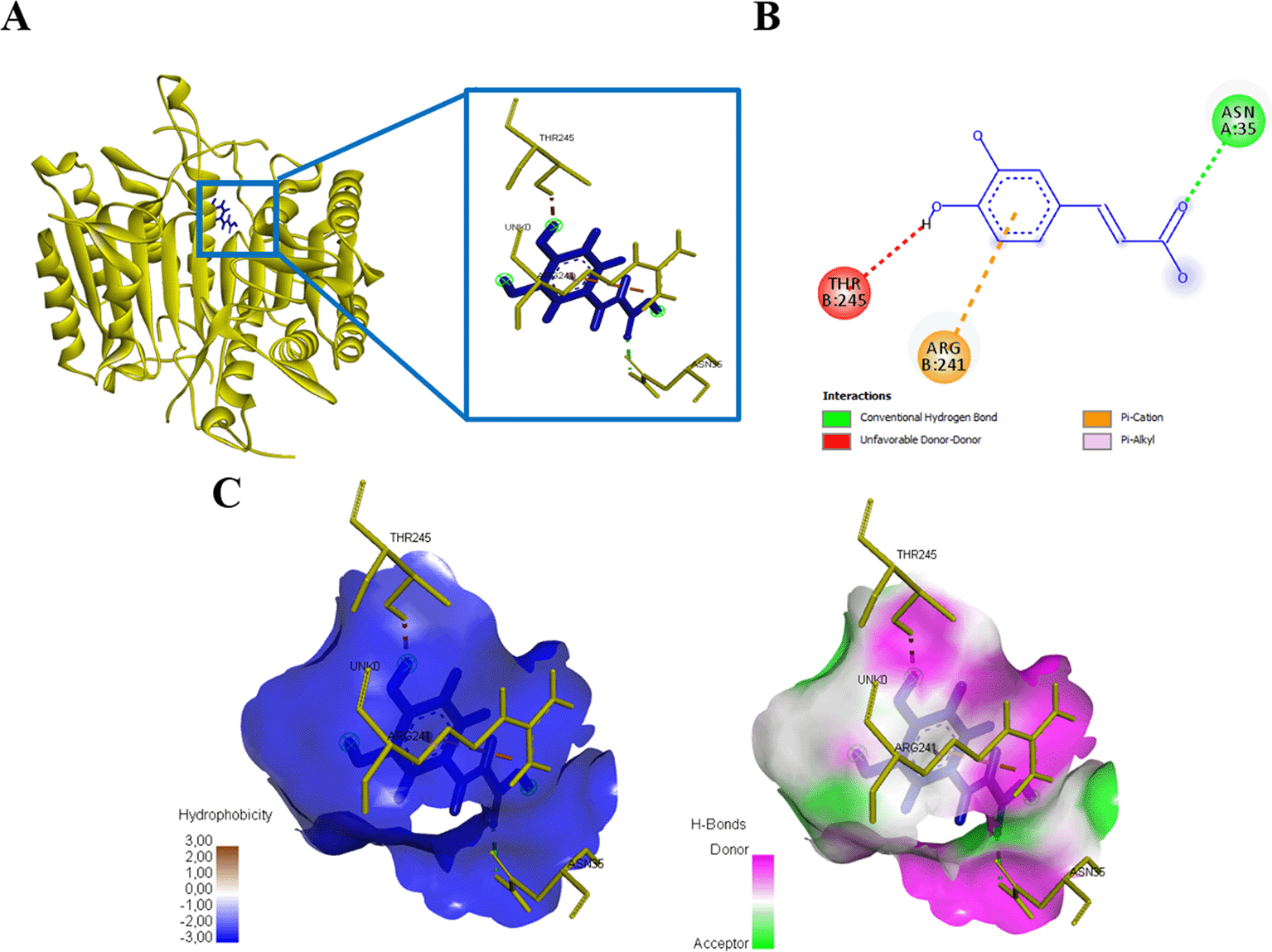
(A) Three-dimensional structural interaction between the ligand Caffeic Acid (blue) and caspase 3 (yellow). This interaction between Caffeic Acid on the active site of caspase 3, called, ASN35, ARG241, THR245, which can be seen in the two-dimensional (B) and three-dimensional structures (C).
(A) Three-dimensional structural interaction between the ligand Caffeic Acid (blue) and caspase 9 (green). This interaction between Caffeic Acid on the active site of caspase 9, called, GLU161, which can be seen in the two-dimensional (B) and three-dimensional structures (C).
Rosmarinic acid docked with protein caspase 3 forms an energy affinity of -265.8 kJ/mol. This bond indicates the presence of amino acid residues that contribute to binding rosmarinic acid compounds, namely ARG164, CYS264, GLY165, GLU124, PRO201, VAL266, LYS137 (Figure 10, Table 3). Rosmarinic acid binds caspase 9 at the amino acid residue ARG341 have an energy of -180 kJ/mol (Figure 11, Table 3).
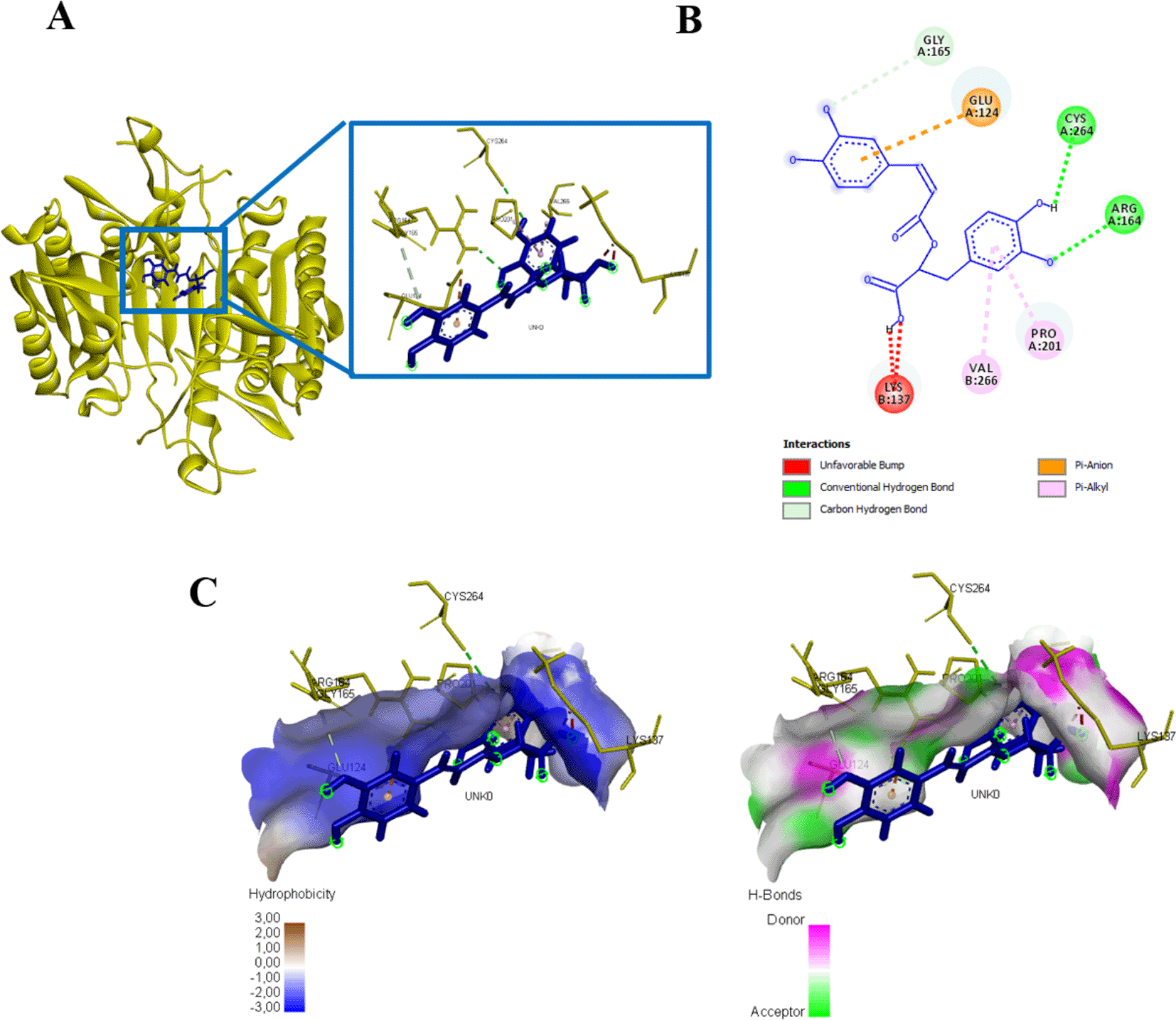
(A) Three-dimensional structural interaction between the ligand Rosmarinic Acid (blue) and caspase 3 (yellow). This interaction between Rosmarinic Acid on the active site of caspase 3, called, ARG164, CYS264, GLY165, GLU124, PRO201, VAL266, LYS137, which can be seen in the two-dimensional (B) and three-dimensional structures (C).
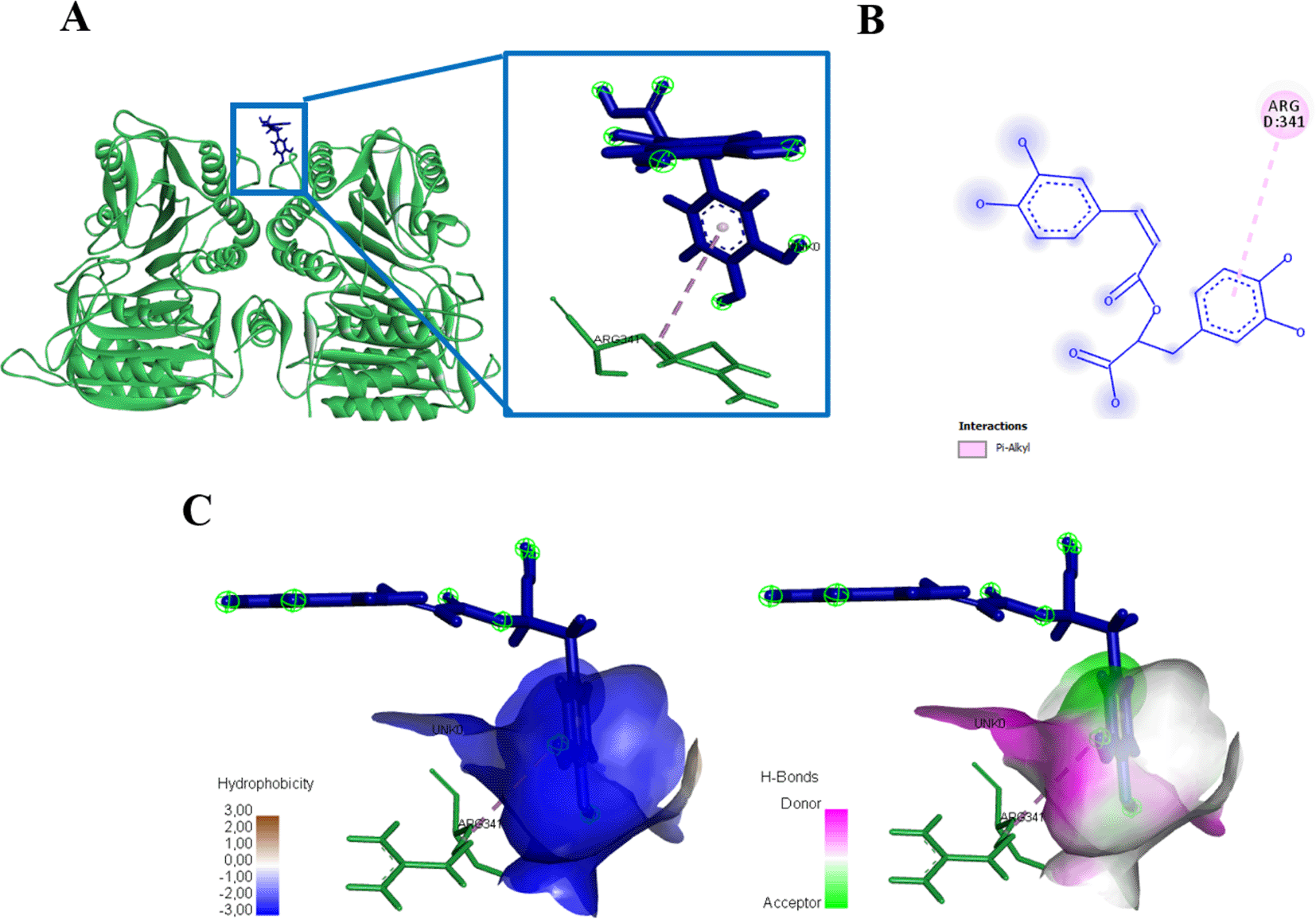
(A) Three-dimensional structural interaction between the ligand Rosmarinic Acid (blue) and caspase 9 (green). This interaction between Rosmarinic Acid on the active site of caspase 9, called, ARG341, which can be seen in the two-dimensional (B) and three-dimensional structures (C).
Interaction results between caspase 3 and Apigenin show there are three amino acid residues binding apigenin namely PHE247, TRP241, dan LYS242 with energy -245.3 kJ/mol (Figure 12, Table 4). Meanwhile, there is no binding between caspase 9 and apigenin, but still produces energy -145.2 kJ/mol (Figure 13, Table 4).
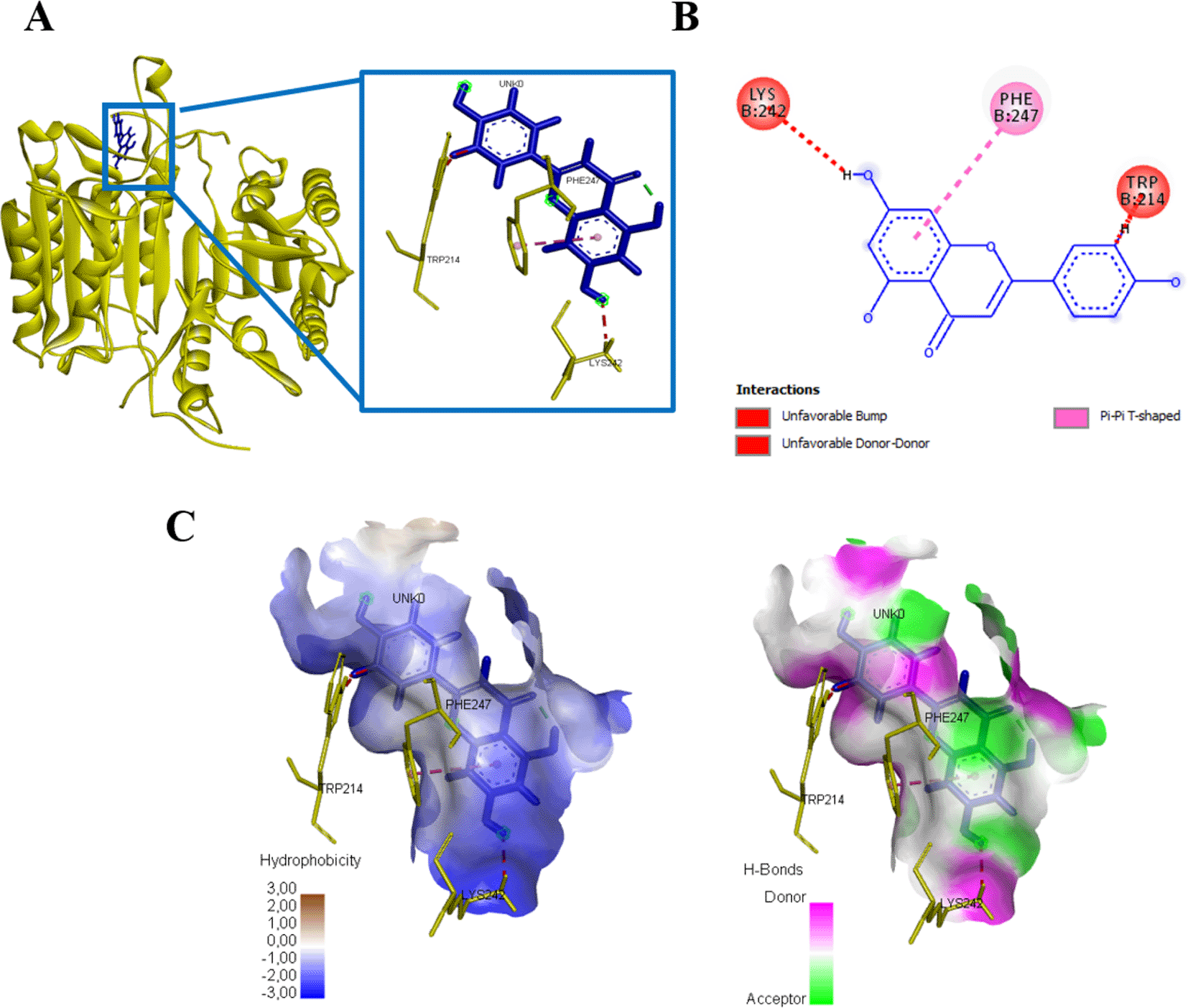
(A) Three-dimensional structural interaction between the ligand Apigenin (blue) and caspase 3 (yellow). This interaction between Apigenin on the active site of caspase 3, called, PHE247, TRP241, dan LYS242, which can be seen in the two-dimensional (B) and three-dimensional structures (C).
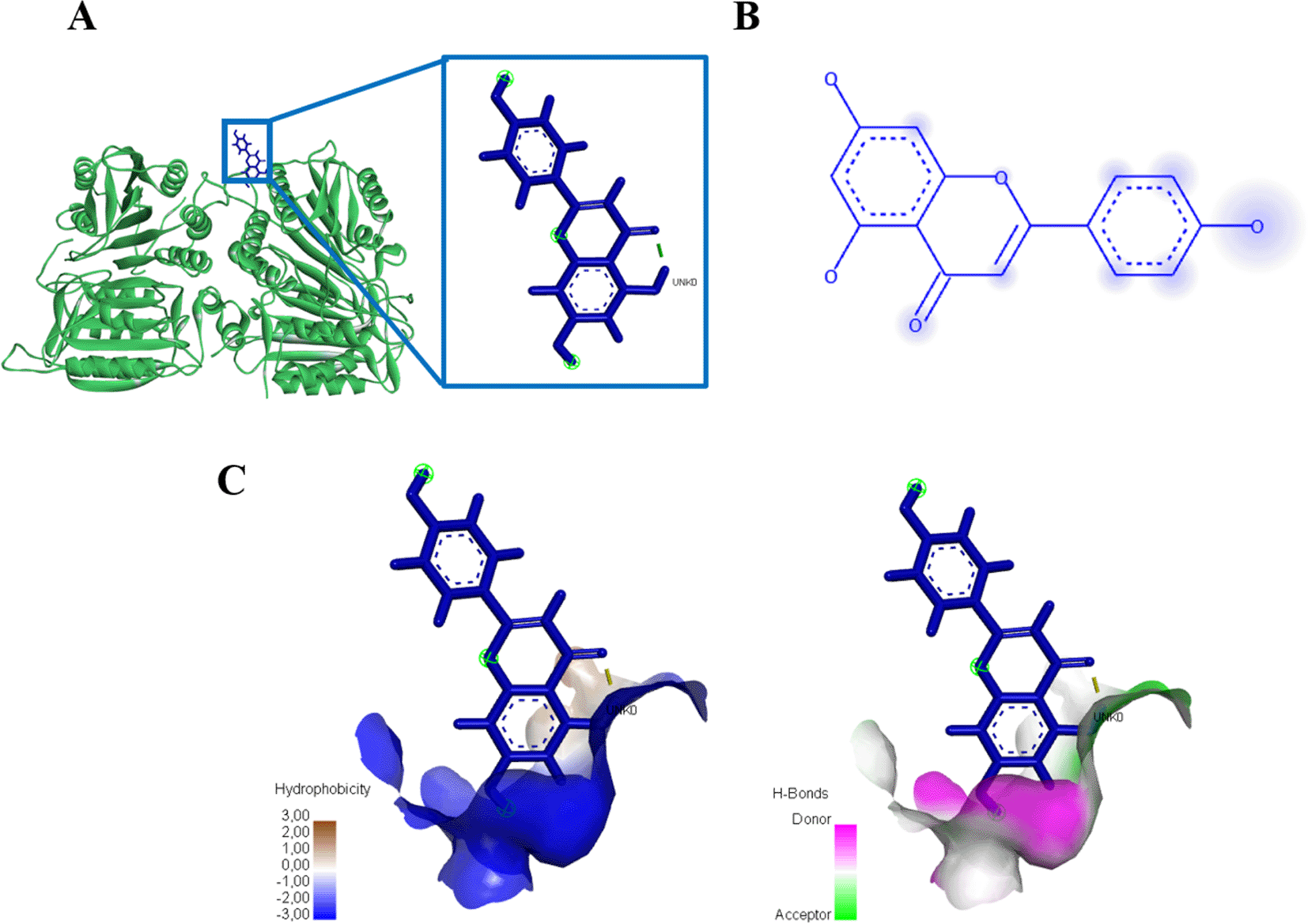
(A) Three-dimensional structural interaction between the ligand Apigenin (blue) and caspase 9 (green). There is no interaction between Apigenin on the active site of caspase 9, which can be seen in the two-dimensional (B) and three-dimensional structures (C).
Currently, breast cancer treatment requires collaborative efforts among several treatment methods. Chemotherapy shows extraordinary efficacy in destroying cancer cells but causes side effects, such as nausea and vomiting, diarrhea, constipation, and alopecia, to impaired immune function. Side effects' severity depends on the drug type, dosage, duration of treatment, and the patient's condition.14 For centuries, herbal plants and their extracts have been used as food and medicine. This review concerns various plants that retain their anti-tumour properties.15 Therefore, herbal medicines are popular, so scientific studies are needed to prove them. It has been reported that Ocimum sanctum is an herbal plant with many health benefits. Studies have revealed the potential of EEOS as an anticancer drug candidate. EEOS can induce apoptotic activity through the mitochondrial pathway12 and inhibit angiogenesis of the human lung adenocarcinoma cell line A549,13 EEOS inhibits invasion and reduces the activity of MMP-2 and MMP-9 HN4 and carcinoma head and neck squamous cells HN12,16 EEOS has cytotoxic ability against leukemia cells K56217 and oral cancer cell line Ca9-22.18
In this study, EEOS demonstrated a significant antiproliferative effect against the MCF-7 and T47D human breast carcinoma cell lines. The MTT assay evaluated the cytotoxic efficacy of O. sanctum. The results showed that the number of surviving MCF-7 cells was significantly lower when treated with various forms of extract compared to the control (Figure 1A). On the other hand, T47D cell viability decreased significantly at a concentration of 375 (μg/ml) (Figure 1B). When the dose-dependent effect was evaluated, all groups showed a steady decrease in the percentage of surviving cells. The ability of EEOS to inhibit the adhesion of MCF-7 and T47D cells were tested using the CCK-8 assay and it was seen that the viability of MCF-7 and T47D cells decreased in line with increasing EEOS concentration (Figure 2A, B). This finding could be interpreted as increasing the concentration of EEOS, the cytotoxic activity and adhesion of the extract increased stably with a significant correlation.
Morphological evaluation of breast cancer cell lines treated with EEOS using SEM revealed the positive cytotoxic ability of EEOS. The morphological changes of the two cell lines, which are characteristic of apoptosis, showed the anticancer activity of O. sanctum leaves with ethanolic extract (Figures 3, 4). According to Yulianto et al. (2017),19 the morphology of cells given anticancer compounds will experience (cell shrinkage) and become denser, and cell organelles will be more tightly packed. Haryanti and Widi-yastuti (2017)20 added that changes in cell morphology in the form of differences in cell dimensions (shrinkage), cytoplasmic compaction, and extracellular matrix damage indicate the characteristics of cells undergoing apoptosis. To clarify the results obtained, we measured the levels of NO) with the Griess test. NO is a ubiquitous water-soluble gas with free radicals. NO also plays a role in inflammatory and immune responses. However, the role of NO in tumors needs to explore deeply. A recent study conducted on the NO has been shown to have anticancer effects. These effects depend on the time, location, and concentration of NO in cells.21 This study showed that on incubation of MCF 7 and T47D cells treated with EEOS, produces higher the NO in line with higher EEOS concentration (Figure 5). NO has been studied as a potential promoter of breast cancer, and increased concentrations of NO were detected in the blood of breast cancer patients.22 An increase in the amount of NO was observed in MCF-7 cells treated with the methanol extract of Fraxinus micrantha.23 NO is produced in a concentration and time-dependent manner. The expression of several genes involved in tumor biology and tumor development is regulated by NO and is largely regulated by NO-mediated posttranslational modification (PTM) protein. The affected proteins cause cellular dysfunction that contributes to cancer onset, growth, development, invasion, and metastasis.24
Kumar and Kashyap (2015)23 stated that an increase in NO levels could occur due to the administration of various agents that cause apoptosis. A wide variety of active phytochemicals, such as flavonoids, phenols, terpenoids, saponins, alkaloids, steroids, and tannins have been identified in EEOS.25 EEOS phytochemical compounds can act as antiproliferative and apoptotic agents in NSCLC cells by in silico molecular docking and in vitro experiments with different mechanisms of action.26 Studies showed that the phytochemicals, quercetin, and genistein (flavonoid members), epigallocatechin-3-gallate (phenol members), and sulforaphane induce NO production, alter the redox environment, and thereby facilitate apoptosis. NO is produced by three NOS enzymes. The mRNA transcript levels of the three NOS enzymes after phytochemical treatment were assessed, and it was shown that after treatment with all phytochemicals, the expression of two or more NOS transcripts increased. Furthermore, the protein levels of iNOS and eNOS were found to be significantly increased.27
In former research, the ethanolic extract of Ocimum sanctum Linn. contains active compounds of flavonoids, phenols, alkaloids, tannins, saponins, terpenoids, and steroids.28 The active compounds in plants have anticancer effects and are used as therapeutic agents with low toxicity. In this study, Gallic acid (GA) is a natural phenolic compound that has a synergistic anticancer effect with paclitaxel and carboplatin to reduce proliferation. These effects are mediated through inhibition of the mitotic cycle, increased apoptosis, and overexpression of P53, Bax, and caspase-3 in breast cancer29 and cervical cancer.30 Caffeic acid (CA) is an antioxidant in normal cells, and pro-oxidant cells in tumor cells have low toxicity in normal cells and can inhibit cancer growth. Caffeic acid can suppress cell proliferation in breast cancer by mimicking antiestrogen activity and regulating growth regulators Estrogen Receptor (ER)/cyclin D1 and Insulin-Like Growth Factor I Receptor (IGF-IR)/pAkt, thereby leading to the development of cell cycle progression of damaged cells and decreased cell proliferation (Rosendahl et al., 2015), re-ducing IL-2 and activating NF-κB (Chen et al., 2018) and apoptotic ability through the intrinsic mitochondrial pathway.31,32
Rosmarinic acid (RA) also has phenol bioactive properties as an anticancer. RA inhibits cell proliferation and induces apoptosis in breast cancer and cervical cancer through cell cycle studies and apoptosis33 An article shows that RA has the ability as an anti-inflammatory and antioxidant. As an anticancer, RA can inhibit cell proliferation and migration, induce apoptosis, and as antiangiogenesis, it is expected to prevent tumor growth and metastasis.34 Research by Anwar et al., (2020)35 showed that atomistically, RA can inhibit microtubule regulatory kinase (MARK4) from controlling cell growth and inducing apoptosis. Apigenin, a natural bioflavonoid, is reported having low cytotoxicity and precise anticancer properties. Apigenin regulates signaling and participates in cancer cell metastasis, proliferation, and invasion.36 Apigenin induces apoptosis via an extrinsic caspase-dependent pathway in breast cancer37 and decreases CK2α expression levels in cervical cancer.38 Apigenin is able to induce ROS in MCF-7 intracellular cells thereby triggering the activation of p21 activation to induce termination of G2/M cells by promoting Cdc25C and CDK/cyclin. Moreover, ROS induction is also responsible for p53 activation, followed by activation of the caspase cascade pathway.39 Apigenin also has antiproliferative effects via estrogen receptor signalling and Akt/FOXO3a/FOXM1 signalling.40
In this current study, we used in silico molecular docking approaches to determine the mechanism of action of the EEOS. In cancer, there is an imbalance between cell differentiation and cell apoptosis. Apoptosis has an important role to maintain cellular homeostasis. In the process of apoptosis, caspases have a role in initiation and execution. Interestingly, in this study, we found a novelty in which we found other active sites of caspase bound by compounds of natural origin, namely ASN35, ARG241, THR245, THR166, GLU123, GLU167, PHE247, TRP214, LYS242, ARG164, CYS264, GLY165, GLU124, PRO201, VAL266, LYS137 with hydrogen bonds, Pi-Anion, Pi-Alkyl, Pi-Pi T-shaped, and Pi-Cation. Binding to the active site of caspase 3 affects the activation of caspase 3 and its regulation as a stimulator of apoptosis in cells. On the other hand, the amino acids GLU161, SER194, and ARG341 also bind to the active site of caspase 9 by Pi-Anion, Pi-Alkyl, and hydrogen bonds (Figure 14). In the process of apoptosis, the executor caspase will be activated when cleavage occurs by upstream initiators (including caspase 9) or additional proteases. Caspase 9 has a caspase recruitment domain (CARD)—apoptotic peptidase activating factor 1 (APAF1) apoptosome for caspase-9.41 Apoptosomes recruit and activate caspase-9, which continues the caspase cascade by cleaving caspases-3 and -7. To cleave caspase-3, caspase-9 needs to remain bound to the apoptosome and caspase-9 autocleavage to the p35/p17 subunit induces dissociation from apoptosis. Caspase-9 or caspase-3 in cancer is involved in apoptosis downstream of mitochondrial outer membrane permeabilization (MOMP). These caspase-9 and caspase-3-based therapies can induce MOMP and increase their cytotoxicity.42 Our study demonstrates that EEOS inhibits proliferation of MCF-7 and T47D breast cell cancer by increasing production of Reactive Nitrogen Species (RNS), thus altering the morphology of cells, and ending up with the increasing activity of Caspase-3 and Caspase 9. Further studies to know more deeply the mechanisms of how ethanolic extract Ocimum sanctum Linn. exerts its anticancer activity are required.
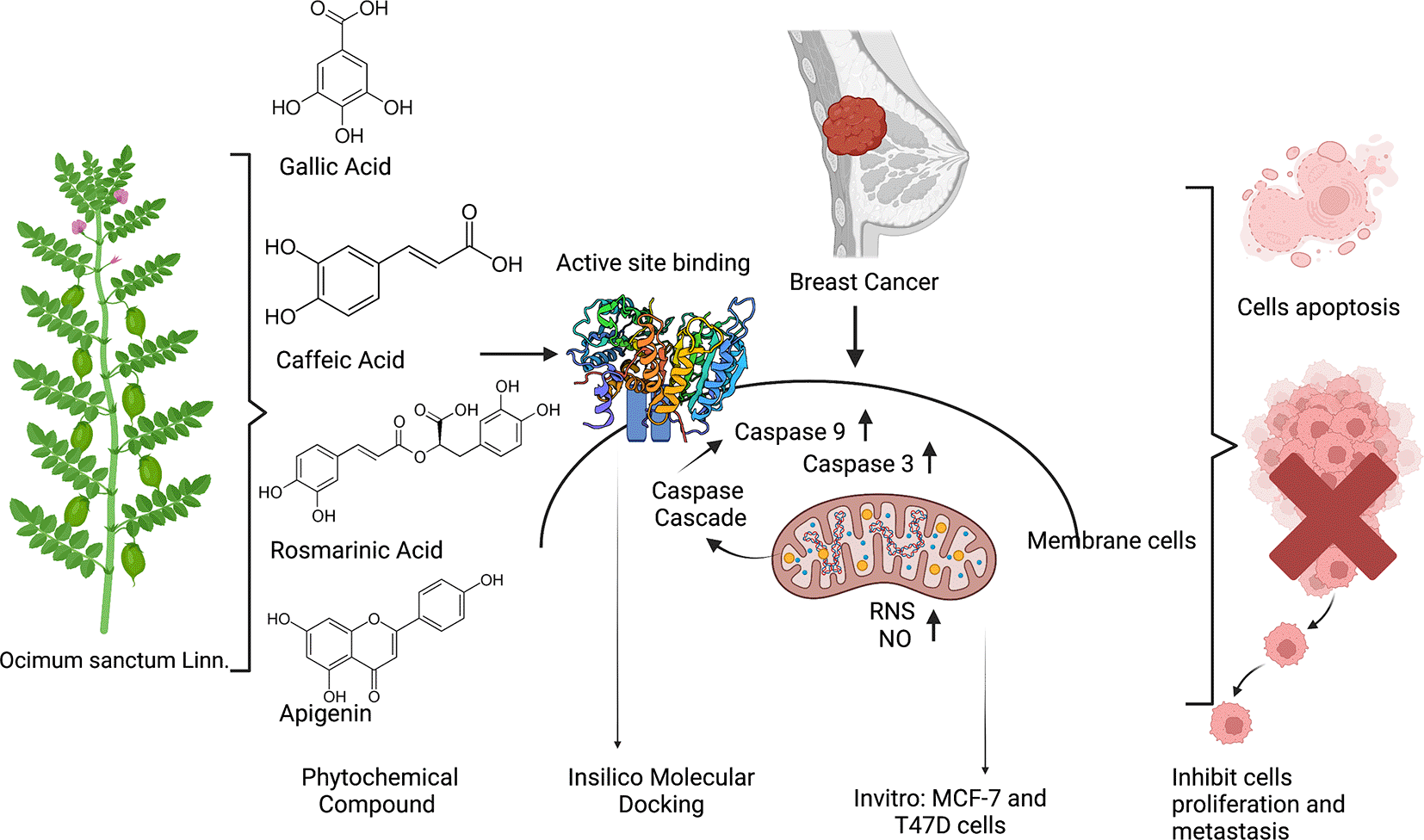
triggers the increasing expression of RNS/ROS in addition the phytochemical also interacts with the active site of Caspase 9 and Caspase 3 thus may increase the expression of Caspase 3 as well as Caspase 9 and push the cells going to apoptosis.
PubChem: Apigenin. Accession number: 5280443, https://identifiers.org/pubchem.compound:5280443. 43
PubChem: Caffeic Acid. Accession number: 689043, https://identifiers.org/pubchem.compound:689043. 44
PubChem: Gallic Acid. Accession number: 370, https://identifiers.org/pubchem.compound:370. 45
PubChem: Rosmarinic acid. Accession number: 5281792, https://identifiers.org/pubchem.compound:5281792. 46
PBD: Structure of cleaved, CARD domain deleted Caspase-9. Accession number: 1jxq, https://identifiers.org/pdb:1jxq. 47
PBD: Caspase-3 tethered to irreversible inhibitor. Accession number: 1nms, https://identifiers.org/pdb:1nms. 48
The authors wish to thank World Class Professor Program 2022, for the facilitation of fine tuning during the preparation of the manuscript. We thank to Integrated Laboratory for Research and Testing for the use of Scanning Electron Microscope, and also Yusuf Umardani, M.Eng., Puspa Hening, M. Biotech, Golda Rani Saragih, D.V.M. for excellent technical assistance.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular Genetics & Human Reproductive Hormones Study, Pharmaceutical Industry, Biotechnology, Medical Pharmacology & Basic Pharmacology (Pharmacokinetic and Pharmacodynamic)
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 24 Mar 23 |
read | |
|
Version 1 06 Feb 23 |
read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)