Keywords
vitamin D; COVID-19; severity; supplementation
This article is included in the Emerging Diseases and Outbreaks gateway.
Background: The aim of the current study was to assess the patients with COVID-19 and the impact of vitamin D supplementation on the course of COVID-19.
Methods: This prospective cohort study included patients hospitalized due to COVID-19 between December 2020 and December 2021. Patients' demographic, clinical, and laboratory parameters were analysed.
Results: 301 participants were enrolled in the study. 46 (15,3%) had moderate, and 162 (53,8%) had severe COVID-19. 14 (4,7%) patients died, and 30 (10,0%) were admitted to the ICU due to disease worsening. The majority needed oxygen therapy (n=224; 74,4%). Average vitamin 25(OH)D3 levels were below optimal at the admittance, and vitamin D deficiency was detected in 205 individuals. More male patients were suffering from vitamin D deficiency. Patients with the more severe disease showed lower levels of vitamin 25(OH)D3 in their blood. The most severe group of patients had more symptoms that lasted significantly longer with progressing disease severity. This group of patients also suffered from more deaths, ICU admissions, and treatments with dexamethasone, remdesivir, and oxygen.
Conclusion: Patients with the severe course of COVID-19 were shown to have increased inflammatory parameters, increased mortality, and higher incidence of vitamin D deficiency. The results suggest that the vitamin D deficiency might represent a significant risk factor for a severe course of COVID-19.
vitamin D; COVID-19; severity; supplementation
The changes are reflecting the comments, questions and observations from both reviewers. We have managed to amend all minor corrections such as changes in the name of Y-axis for all three figures. Furthermore, conclusion section of the manuscript has been re-written in order to encompass all limitations of our research and interpret potentially contradicting results with previously published reports, which in general were different due to different methodology in our research. The main message of our study is that the correlation between vitamin D deficiency and the severity of the COVID-19 disease is less pronounced if vitamin D is supplemented from admission onwards, and mortality is low. Hence, we also improved the interpretation of results, when comparing patients with different levels of vitamin D. Moreover we have more clearly defined our methodology, including missing information for patient with ARI, who was also COVID-19 positive, and defined the duration (time) of symptoms.
See the authors' detailed response to the review by Giulia Vivaldi
See the authors' detailed response to the review by Radek Kučera
Past pandemic waves of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) led to many critical cases and, consequently, deaths of high-risk patients. However, previous studies have shown fluctuations in coronavirus disease 2019 (COVID-19) severity throughout the year, especially during different seasons. Notably, seasons also play a role in immunity variation demonstrated by significant differences in the circulating immune cells such as lymphocytes and neutrophils.1,2 Moreover, studies have shown a strong correlation between low levels of serum vitamin D3 (25-hydroxyvitamin D3, 25(OH)D3) and the incidence of COVID-19,1,2 which coincides with most viral respiratory infections occurring during the winter season.3 Previous studies have shown a positive correlation between COVID-19 severity and vitamin D deficiency. Furthermore, vitamin D supplementation was shown to improve the clinical outcome of COVID-19 patients.4,5 Vitamin D deficiency is a global health problem as many healthy individuals have suboptimal serum vitamin D levels.1–3 Vitamin D has an immunomodulatory role as it decreases the proliferation of Th1 cells and stimulates the proliferation of Th2 cells, enables the secretion of anti-inflammatory cytokines, and thus lowers the production of pro-inflammatory cytokines.6 In addition to immunomodulatory and anti-inflammatory effects, vitamin D can also reduce the risk of respiratory tract infections, especially viral infections, such as COVID-19.7–17 Several studies confirmed the beneficial role of vitamin D in reducing the risk of susceptibility to acute respiratory infection, a protective effect of vitamin D against viral and bacterial respiratory pathogens in healthy, hospitalized, or critically ill patients.8,18–23 Moreover, a positive correlation was found between low levels of vitamin 25(OH)D3 and worse clinical outcomes (increased hospital stay, readmission to hospital, severity, sepsis, and mortality).22–37 Individuals with lower vitamin 25(OH)D3 levels were shown to have an increased risk of SARS-CoV-2 infection, admission to intensive care unit (ICUs), and increased mortality.38–40
Based on these studies, vitamin D supplementation during the period of respiratory infections and in individuals with COVID-19 was recommended by our group in October 2020, after the first wave of COVID-19. The ad hoc Slovenian recommendations for supplementation were as follows: for the healthy adult population, 800 IE to 2000 IE/day from October to the end of April; for high-risk individuals for vitamin D deficiency and respiratory infections 1000 to 2000 IE/day or 10 000 to 14 000/week. This group included patients with chronic diseases, individuals 70 years of age and older, individuals who live with COVID-19 patients in the same household, individuals with high-risk contact with COVID-19 patients, and healthcare workers. Pregnant women were recommended to take of 1500 IE to 2000 IE/day. Newly diagnosed COVID-19 patients were recommended a loading dose of 14 000 IE/day for four days followed by 2000 IE/day onwards. For hospitalized COVID-19 patients, the measurement of 25(OH)D3 was recommended and supplementation according to the measured serum levels was advised.41
Prior to the publication of Slovenian recommendations, many studies were published, where factors that contributed to low vitamin 25(OH)D3 levels and deficiency, including higher age, positive smoking status, obesity with higher body mass index (BMI), lack of sun exposure, and the presence of chronic illnesses (diabetes, malignancies, high blood pressure, gastrointestinal disorders, etc.) were identified. Most people with these risk factors have been shown to be at an increased risk regarding both severity of the disease and mortality from COVID-19.42 Thus, it was hypothesized that optimal levels of vitamin 25(OH)D3 in the early phase of SARS-CoV-2 infection could prevent progression to severe or critical COVID-19 and reduce mortality.3,43 Besides the recommendations, considerable public awareness campaigns and educational interventions have been conducted with the objective to increase individual vitamin D supplementation in Slovenia, especially during the second COVID-19 lockdown in December 2020.44 Therefore, the purpose of our study was to assess the severity of COVID-19 patients, who were admitted to our department and determine the impact of Vitamin D supplementation at the admittance on the clinical course of COVID-19.
We designed a prospective cohort study of COVID-19 patients, hospitalised in the COVID-19 unit of the University medical centre Ljubljana between December 2020 and December 2021. The study was approved by the Slovenian Medical Ethics Committee (Protocol ID: 0120-60/2021/5) and adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from all participants enrolled in the study.
Consecutive patients aged 18 or older, in the acute phase of COVID-19, with a PCR confirmed SARS-CoV-2 were included in the study, and their clinical charts were prospectively reviewed to collect the following relevant demographic, clinical, and laboratory parameters: age, sex, presence of comorbidities (diabetes, arterial hypertension, hyperlipidemia, coronary artery disease, history of stroke), history of smoking, alcohol consumption, the presence of COVID-19 related symptoms (fever, chest pain, cough, anosmia, dyspnoea, diarrhoea, and headache), time from the symptom’s onset or positive PCR to hospitalisation, laboratory parameters, COVID-19 related treatment and outcome. All patients underwent chest X-ray imaging in order to confirm the scale of COVID-19 infection and exclude or confirm COVID-19 associated pneumonia. Patients were divided into four groups based on the COVID-19 disease severity classification: (1) asymptomatic (positive PCR, no symptoms), (2) mild (the presence of symptoms but no shortness of breath, dyspnoea, or abnormal chest imaging), (3) moderate (evidence of lower respiratory disease during clinical assessment or imaging and who have an oxygen saturation (SpO2) ≥94% on room air at sea level) and (4) severe disease (SpO2 <94% on room air at sea level, a ratio of arterial partial pressure of oxygen to fraction of inspired oxygen (PaO2/FiO2) <300 mm Hg, respiratory frequency >30 breaths/min, or lung infiltrates >50%).
COVID-19 infection was determined by RT-PCR during this initial visit. A nasopharyngeal swab specimen for the identification of SARS-CoV-2 was taken and sent to Institute of Microbiology and Immunology to confirm the infection. The patients’ blood pressure was measured at admission and 72 hours after admission. Blood samples were collected on a day of admission to the hospital and transported to the laboratory at the University Medical Centre in Ljubljana, where they were stored at minus 80 °C until analysis. Determination of inflammatory biomarkers, C-reactive-protein (CRP), interleukin-6 (IL-6), procalcitonin (PCT), D-dimer and 25(OH)D3 have been carried out according to a routine protocol using standard laboratory procedures. Patients received COVID-19 specific treatment consisting of oxygen supplementation, dexamethasone, and/or remdesivir according to the treatment guidelines.
The concentration of 25(OH)D vitamin was measured using competitive luminescent immunoassay with the limit of quantification 6 nmol/L (Architect analyser, Abbott Diagnostics, Lake Forest, USA). Vitamin D status was assigned with consideration of serum 25(OH)D3 concentration according to the literature: Deficient below 30 nmol/L, insufficient below 50 nmol/L, non-optimal between 50-75 nmol/L and optimal >75 nmol/L.43,45
The statistical analysis was performed using SPSS 21.0 (IBM Inc., Chicago, USA). Normally distributed variables were expressed as arithmetic mean and standard deviation, and One-Way ANOVA test was used for comparisons between variables. In case of abnormally distributed variables the differences between continuous variables were analysed by the nonparametric Mann-Whitney test. Binary logistic regression model was used to identify risk factors for moderate-to-severe COVID-19 course of the disease. Statistical significance for all tests was determined as p-value below 0.05.
Overall, 301 participants were included in the analysis. The baseline demographic and clinical characteristics of patients are presented in Table 1. There was a similar distribution of female and male patients. The average age was 65 years. Laboratory parameters showed systemic inflammation with increased proinflammatory factors such as CRP, PCT, IL-6. Most patients (77%) had COVID-19 pneumonia detected by chest X-ray imaging. One case of acute respiratory insufficiency (ARI) was determined, who was also SARS-CoV-2 positive. 46 (15,3%) patients had moderate, and 162 (53,8%) patients had a severe course of COVID-19. Most patients were admitted to the hospital during winter season. 14 (4,7%) patients died during the follow-up, 30 (10,0%) were admitted to the ICU due to the disease worsening. A vast majority needed oxygen supplementation (n=224; 74,4%).
Average vitamin 25(OH)D3 levels were below optimal, and vitamin D deficiency and insufficiency was detected in 205 (68.1%) individuals. Approximately one third of patients had optimal 25(OH)D3 levels above 75 nmol/L. Serum vitamin 25(OH)D3 levels were significantly lower in winter months (November-April) (p<0.001) (Figure 1). Higher levels of serum vitamin 25(OH)D3 were present in the summer months (Figure 2).
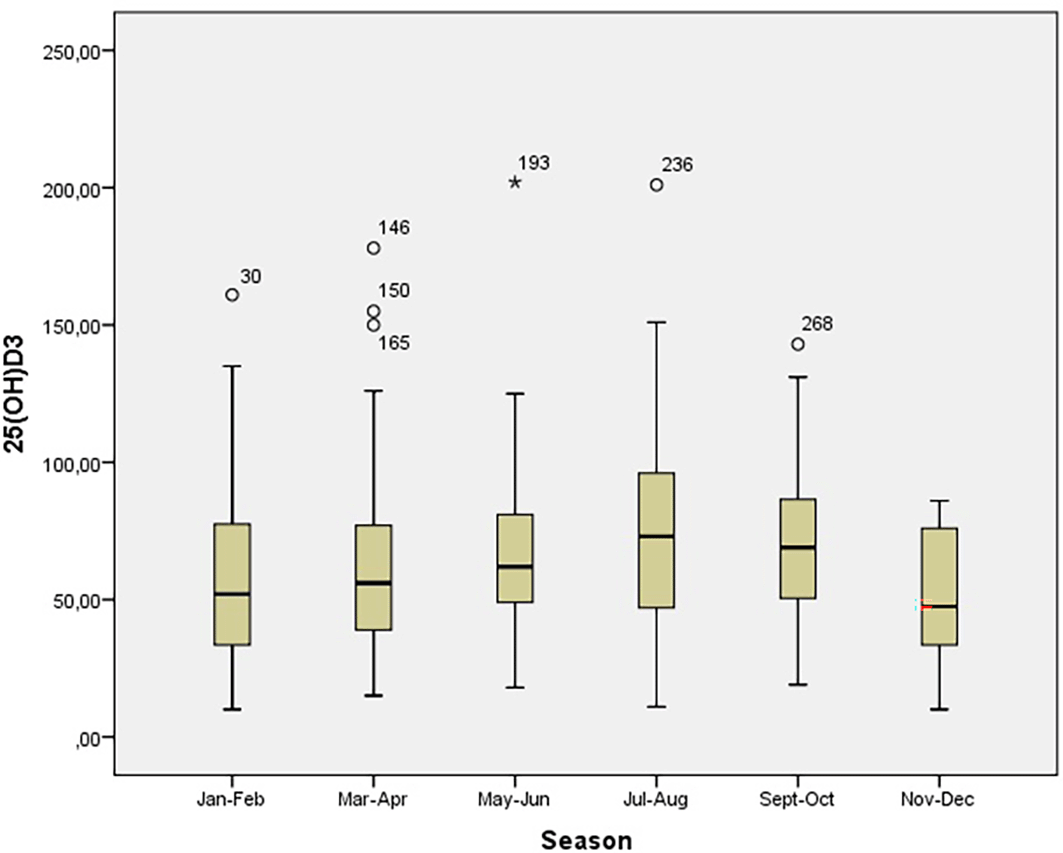
*Measurements with numbers present extreme values.
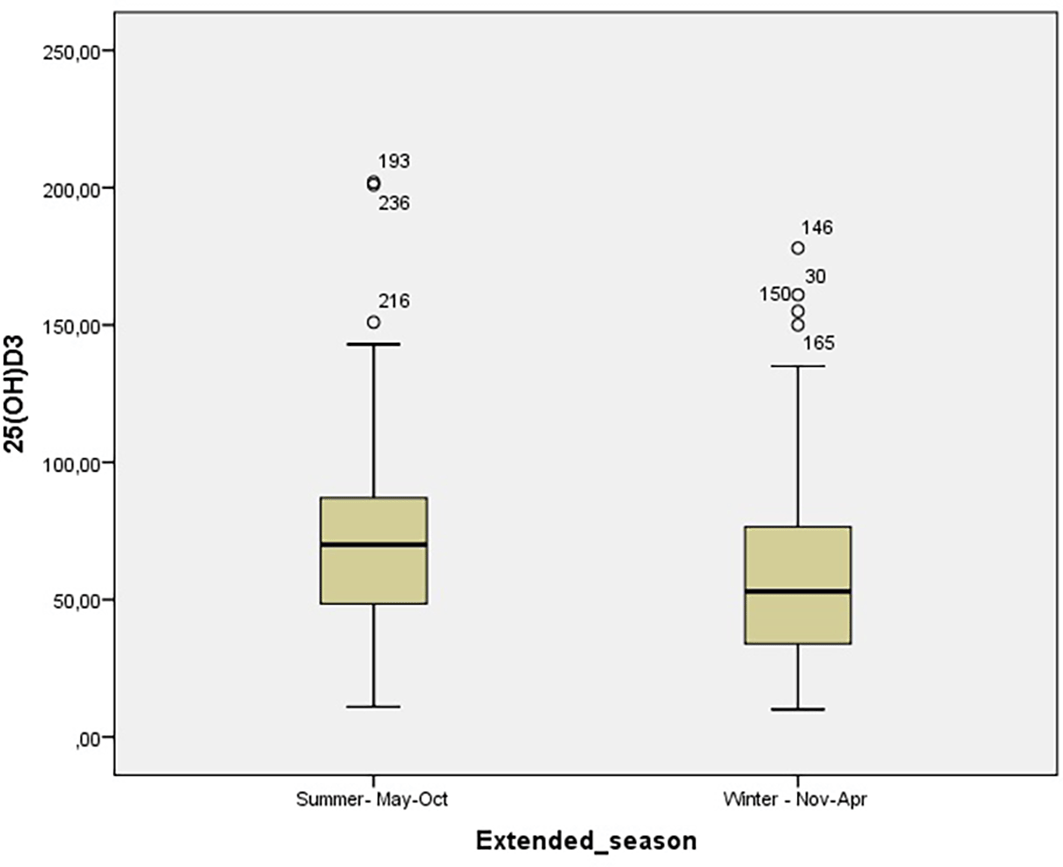
*Measurements with numbers present extreme values.
Patients were divided into four groups based on their serum vitamin 25(OH)D3-deficient, insufficient, sufficient and non-optimal. The difference is clinical characteristics between the groups are presented in Table 2. Vitamin D deficiency was more common in males. A shorter duration of symptoms before hospitalisation was seen in the most deficient group. Duration of symptoms was the time from the first onset of symptoms to admission to the COVID-19 unit. Unfortunately, time was not taken into account for correlation. Fever and cough were more frequent with the non-optimal group of patients. In addition, severe disease was least common in patients who were vitamin D deficient. Group of patients with optimal vitamin 25(OH)D3 levels have experienced more frequent pneumonia so they needed dexamethasone and oxygen therapy.
Table 3 presents the distribution of patients according to the severity of COVID-19. There were notable differences in proinflammatory markers between the groups for LDH, ferritin, CRP, PCT. Patients with more severe disease had increased levels of proinflammatory markers, and significantly lower levels of serum vitamin 25(OH)D3. However, the lowest levels of vitamin 25(OH)D3 were detected in the asymptomatic group (Figure 3). Asymptomatic means that they had no symptoms when admitted to the COVID-19 ward. Patients with severe COVID-19 exhibited more symptoms, had a higher rate of ICU admissions and mortality.
This study aimed to assess the severity of COVID-19 among our patients and potential effect of serum levels of vitamin D levels on the course of COVID-19. Previous studies have speculated about the role of vitamin D supplementation and COVID-19 but have been inconclusive. However, it is known that vitamin D plays an important role in immune system and can improve natural resistance to acute viral respiratory infections and moderate their course by inhibiting an excessive inflammatory response. A high prevalence of vitamin D deficiency especially pronounced in the autumn and winter months was reported in Slovenia, with up to 80% of adults exhibiting serum vitamin D levels <50 nmol/L, and severe deficiency with serum vitamin D levels <30 nmol/L was found in 40%.43 Therefore, our group advised vitamin D supplementation to mitigate the course of the COVID-19 disease, especially in patients at risk for a severe course. Our results show that most enrolled patients were still vitamin D deficient despite the recommendations which were published prior to the conduction of this study. It is noteworthy that vitamin D deficiency was predominantly observed during the winter months from November till April, which confirms our previous findings.43 These results could be explained by mentioned cross-sectional Slovenian study, which showed that at the end of 2020, according to estimates, up to 56% of the Slovenian population was taking vitamin D and/or increased sun exposure in the summer, which is known to be an important source of natural vitamin D. It has been established that in the absence of UVB-induced vitamin D biosynthesis, vitamin D supply can be maintained in healthy adults with a dietary intake of between 600 and 800 IU of vitamin D.46,47 At the same time, it should be pointed out that this does not apply to individuals who already have vitamin D deficiency, and that such doses are sufficient to reach the threshold value for skeletal and muscle functions (50 nmol/L), but not to reach higher concentrations of serum level, e.g. 75 nmol/L. The results of our study show, that the Vitamin D deficiency is more prevalent in males. Interestingly, the duration of symptoms was the shortest in the most Vitamin D deficient group. However, duration of symptoms was only measured as a time between onset of symptoms and admittance to hospital, speculating that the severity of the diseases might be so severe, the patients needed shorter time to visit hospital. Unfortunately we did not measure severity of the disease and the time to admittance to the hospital. Furthermore, fever and cough were more frequent within the group with non-optimal serum Vitamin D levels. In addition, severe disease was least common in patients who were vitamin D deficient. These results do not support the protective role of vitamin D in viral infections suggested in the previous studies, where vitamin D supplementation was shown to significantly lower the incidence of acute respiratory infections (ARIs) in subjects with severe vitamin D deficiency.48 Our results can be explained by several hypotheses. First, previous studies have shown that higher serum levels of vitamin D are required for beneficial effects on the immune system than for skeletal and muscle effects.49 This is further supported by a study in which patients with levels of vitamin D below 75 nmol/L were shown to 58% more likely to suffer from ARI.50 Most of our patients admitted they only started supplementing Vitamin D after they had already developed the symptoms, which could explain our results, as the immunomodulatory effects of Vitamin D have not yet started. Previous studies have shown a correlation between lower average vitamin D levels and a higher incidence of SARS-CoV-2 infections and higher mortality (Italy, France, Spain, Switzerland).51 For instance, in Chicago, those with vitamin 25(OH)D3 levels below 50 nmol/L had a 1.77 times greater risk of testing positive for the infection. Their vitamin 25(OH)D3 was determined up to a year before they fell ill or were tested.52 In our case unfortunately we did not measure levels of vitamin 25(OH)D3 at several time points. Similar differences in the vitamin D levels between positive and negative subjects was also noted in a Swiss study, where those with a positive test had an average level of only 27 nmol/L,53 and those with a negative test had an average level of 61 nmol/L. Also in Israel, it was shown that a lower level of vitamin D increases the risk of infection with SARS-CoV-2.54 In observational studies in Iran,55 Germany, and Italy, those patients with COVID who had lower levels of vitamin D had worse outcomes.56 Moreover, an almost 15-fold increased risk of death was observed in patients with vitamin D deficiency compared with those with higher vitamin 25(OH)D3 levels; similar relationships were also evident when the vitamin 25(OH)D3 cut-off was set at 50 nmol/L.56 Our results show that patients with more severe disease showed higher levels of inflammatory markers and significantly lower levels of serum vitamin 25(OH)D3. Interestingly however, again the lowest levels of vitamin 25(OH)D3 were detected in the asymptomatic group. This can be explained by the same reason as above those patients who had symptoms started taking high concentrations of vitamin D prior to their admission to the hospital as our recommendations were published over several media and in a public campaign. Patients with asymptomatic course of the disease were not aware of infection and were not supplementing vitamin D, which explains the lower levels. In addition, the majority of cases were detected during the winter months, during which the baseline serum Vitamin D levels are lower, which could have influenced the results. Nevertheless, the most severe group of patients exhibited more symptoms, a higher rate of ICU admission and a higher mortality rate which is in line with previous reports. The results of a previous study indicate that vitamin D supplementation decreases the rate of ICU admissions as well as mortality.57 The favourable outcome of vitamin D supplementation and increased serum levels can be explained by the fact that vitamin D moderates the excessive inflammation in the lungs (cytokine storm) that can have detrimental effects in some patients with COVID-19. Inconsistency in the effect of vitamin D supplementation has been shown previously and attributed to different dosing regimens, duration of supplementation, and methodology in published reports. Murai et al.58 did not detect differences in hospital stay of patients with COVID-19 who received vitamin D dose and those who did not receive vitamin D.
Our study had several limitations. No clear data about the length of vitamin D supplementation prior to hospitalisation could be obtained. Our largest limitation is that no follow-up was performed, as in the following period the severity of the disease might be lower due to higher level of serum vitamin 25(OH)D3. In addition, we did not measure the levels of vitamin 25(OH)D3 in several time points of hospitalisation to confirm its effect over time. Most studies to date suggest that vitamin D deficiency and the severity of the course of the COVID-19 disease correlate. In our study, the results are different and somewhat contradictory. The conflicting results are most likely due to in-hospital vitamin D supplementation in those who had severe vitamin D deficiency or insufficiency, with the course also being mostly mild in the severe vitamin D deficiency and vitamin D deficiency groups, as well as overall mortality in all groups very low compared to other studies.
Our study explored the relationship between vitamin D levels and COVID-19 severity. We observed that patients with severe COVID-19 had increased inflammatory markers, higher mortality rates, and a higher incidence of vitamin D deficiency. Notably, our findings also revealed that the lowest levels of serum vitamin 25(OH)D3 were found in asymptomatic patients. This seemingly paradoxical result aligns with the hypothesis that vitamin D receptors in immune cells might play a role in modulating immune responses. A decrease in serum 25(OH)D3 could indicate an effective immune response, preventing the progression to severe disease. However, this observation requires further investigation.
The exact mechanism of vitamin D in viral infections remains incompletely understood. Our study had limitations, including gaps in the history of vitamin D use before hospitalization. Despite these limitations, our findings contribute to the growing body of evidence suggesting that vitamin D deficiency may be a significant risk factor for severe COVID-19. The main message of our study is that the correlation between vitamin D deficiency and the severity of the COVID-19 disease is less pronounced if vitamin D is supplemented from admission onwards, and mortality is low. Therefore, we recommend vitamin D supplementation in high-risk individuals and hospitalized COVID-19 patients. Future prospective longitudinal studies are warranted to fully understand the long-term effects of vitamin D supplementation and its role in the immune response to SARS-CoV-2.
D.Si. and J.O. conceived of the idea for the project; D.Si. and J.O. wrote the draft version of the manuscript, R.S. supervised the patients invited to participate in the study; J.U. and K.J. compiled the clinical data on the patients involved; O.J. and D. St were responsible for completing laboratory data, and D.St. and R.J. performed the statistical calculations. All authors reviewed and agreed with the manuscript, and final approval was given by D.Si. and J.O. All authors have read and agreed to the published version of the manuscript.
Zenodo: Siuka, Darko, Saletinger, Rajko, Uršič, Jure, Jevnikar, Kristina, Janša, Rado, Štubljar, David, & Osredkar, Joško. (2023). The effect of Vitamin D levels on the course of COVID-19 in hospitalized patients – a 1-year prospective cohort study (1.0) [Data set]. Zenodo. DOI: https://doi.org/10.5281/zenodo.7679129. 59
Data are available under the terms of the Creative Commons Attribution 2.0 Generic.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: immunochemistry, pharmacology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: immunochemistry, pharmacology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Cohort study analysis
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 2 (revision) 03 Jul 24 |
read | |
|
Version 1 09 Mar 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)