Keywords
Salinity Stress, Antioxidants, Polyamines
This article is included in the Plant Science gateway.
This article is included in the Cell & Molecular Biology gateway.
Salt stress, a significant environmental problem was studied in barley cultivars Giza 124 and Giza 119 at various stages (seedling, pre-flowering, and yield). This study aimed to investigate the impact of salt stress on these cultivars, examine the effects of polyamine precursors (arginine, methionine, and ornithine) on their response to salt stress, and assess the efficacy of antioxidants (glutathione and ascorbic acid) in alleviating the harmful effects of salt stress on barley plants.
Barley grains were germinated and subjected to salinity stress, with subsequent treatment using glutathione, ascorbic acid, or an amino acid mixture. Growth criteria, photosynthetic pigments, metabolites, antioxidant enzymes, mineral content, and polyamines were analyzed.
The impact of 100Mm NaCl, with or without glutathione, ascorbic acid, or amino acid mixtures, on various physiological parameters in G124 and G119 were investigated. The levels of chlorophyll a, chlorophyll b, and carotenoids significantly varied under different treatments. For instance, chlorophyll a in G 124 exhibited a 23% reduction under salt stress compared to the control, while the addition of glutathione mitigated this effect, resulting in a 17% increase compared to the NaCl treatment. Similar trends were observed for chlorophyll b and carotenoids. At the yield stage, both cultivars demonstrated a significant decrease in the the weight of grains per plant under salinity, which was alleviated by the addition of ascorbic acid, glutathione, or amino acid mixtures.
The application of glutathione, ascorbic acid, or an amino acid mixture mitigated the adverse effects of salt stress on various parameters. The results highlight the potentail of these compounds in enhancing plant tolerance to salinity stress and offer insights into the physiological response of barley cultivars under adverse conditions.
Salinity Stress, Antioxidants, Polyamines
Following recommendations from reviewers, several improvements were incorporated to enhance the manuscript. First, a comprehensive modification was made to the abstract, adding new information and improving language according to the reviewers suggestions. The introduction was strengthened by adding the objectives with a more extensive range of references to support the context.
In the methodology section, the initial part was reworded to enhance clarity regarding the treatments and align the pigment approach more cohesively. Furthermore, a detailed overview of the statistical analysis process was provided.
Updates with more information about the results and perspectives from previous research were added to the discussion section, enriching the discussion as a whole.
The conclusion section was rephrased, incorporating perspectives for future research directions to provide a more forward-looking conclusion.
More citations were incorporated. Additionally, more keywords were added and categorized alphabetically for better search engine performance.
See the authors' detailed response to the review by Chandra Shekhar Seth
See the authors' detailed response to the review by Nasim Ahmad Yasin
Salinity is one of the most prevalent abiotic pressures in arid or semi-arid settings (El Sabagh et al., 2020; Hussain et al., 2019; Koca et al., 2007). High salinity negatively affects plants as a whole, resulting in a reduction in productivity or plant death (Van Zelm et al., 2020). Furthermore, reports suggest that agricultural soil salinization is increasing at a rate of 10% per year. By 2050, this tendency might cause more than 50% of arable land to become salinized (Sheoran et al., 2022). Salinity stress disrupts physiological processes in plant cells, inhibits plant growth, and causes significant damage to photosynthesis (Arif et al., 2020; Etesami & Glick, 2020; Shahid et al., 2020; Shang et al., 2022). Yan et al. (2018), resulting in declines in plant production quantity and quality (Taize & Zeiger, 2006). Furthermore, salinity has impacted plant growth and development by increasing salt content, particularly ionic chloride Cl and sodium Na+, leading to changes in water status, mineral uptake, stomatal behavior, carbon allocation, and ion imbalance, resulting in water and osmotic potential disturbance (Hasanuzzaman et al., 2013). Salt stress reduces stomatal conductance, transpiration rate, and intercellular CO2 concentrations by decreasing photosynthesis (Khoshbakht et al., 2018; Xu et al., 2018). Many strategies have evolved by plants to overcome the ionic and osmotic stresses brought on by salinity. Plants that tolerate high salinities have improved osmotic adjustment capacities, which enable them to effectively absorb water.
Barley plants exhibit distinctive responses to salt stress, characterized by the synthesis of specific biochemical compounds and the activation of molecular systems at both cellular and plant levels (Li et al., 2019). Unlike some other cultivated plant species, barley demonstrates a nuanced reaction to salt stress, with variations in survival and productivity outcomes after prolonged exposure to salt. Yancey et al. (1982) reported that salt stress induced physiological and biochemical changes in the whole plant so that various compounds are synthesized such as abscisic acid, organic acids, proline, and polyamines (PAs) (De Oliveira et al., 2020; Gupta et al., 2013). Polyamines are phytohormones that can be utilized to protect plants from abiotic stresses and to induce flowering (Arif et al., 2020; Liu et al., 2015). Because PAs levels change significantly in response to environmental stress, it has been reported that they are part of a plant’s stress defense mechanism (Turano & Kramer, 1993). Polyamines are involved in various plant development processes, including cell division, embryogenesis, floral and reproductive organ development, fruit ripening, root growth, leaf senescence, and response to biotic and abiotic stressors such as salt stress (Agudelo-Romero et al., 2013; Pál et al., 2015; Sarwat et al., 2013; Tyagi et al., 2023). Zhao and Qin (2004) suggested that polyamines improve all morphological and physiological characteristics and prevent chlorophyll degradation while also increasing the accumulation of all organic compounds under salt stress (Khoshbakht et al., 2018). In recent years, attention has been focused on the possible roles of the conjugated forms of polyamines in plants exposed to unfavorable environmental conditions (Khoshbakht et al., 2018; Yousefi et al., 2021; Bouchereau et al., 1999), but even so, only a few reports on the response of bound polyamines to salinity stress factors are available.
Reactive oxygen species (ROS) have become widely recognized to be important in causing a diverse range of stress-induced damage to macromolecules, which eventually affects the structure of cells (Sairam et al., 2002). As a result, the role of antioxidant enzymes such as superoxide dismutase (SOD), ascorbate peroxidase (APX), glutathione reductase (GR), and catalase (CAT), as well as metabolites such as ascorbic acid, glutathione, -tocopherol, flavonoids, and carotenoids (CAR), in the quenching of ROS becomes critical (Ahmad et al., 2018; Noctor et al., 2016; Gupta & Seth, 2020; Zrig et al., 2015; Soliman et al., 2019). Non-enzymatic antioxidants (glutathione and ascorbate) accumulated in root tissues of plants treated to salt stress, according to Vaidyanathan et al. (2003). Glutathione (GSH) is the most abundant non-protein thiol found in animal, plant, and bacterial cells. It contributes to the structural integrity of cells and has the potential to be utilized in the redox control of cell division (Hossain et al., 2017). Glutathione’s physiological importance in plants comes from its role in sulfur metabolism and defense, where it is a vital source of reduced sulfur (Capaldi et al., 2015; Hasanuzzaman et al., 2017; Smirnoff & Wheeler, 2000). It controls a wide range of crucial plant processes including photosynthesis, DNA biosynthesis and repair, protein biosynthesis, amino acid transport, and enzyme control and activation. GHS has recently been discovered to play an important role in protecting chickpea plants from drought stress by regulating the antioxidant defense system and osmolyte synthesis (El-Beltagi et al., 2020).
According to Khan et al. (2011), ascorbic acid (AA) is one of the most significant and abundant growth promoters found in plants. A small amount of AA produced endogenously is involved in the promotion of the development and growth of plant cells. Along with development and growth, ascorbic acid is linked to a variety of environmental stress conditions and involves in phytohormonal-driven signaling pathways (Akram et al., 2017; Barth et al., 2006).
Given the limited understanding of the involvement of polyamines in the salt tolerance of grain crops, the current study aims to investigate the impact of polyamine precursors—arginine, methionine, and ornithine—as well as the influence of antioxidants such as glutathione and ascorbate on the growth, metabolism, and productivity of two barley cultivars with varying salt tolerance when exposed to salt stress.
The objectives of this study include the following:
1. Investigate the Impact of Salt Stress on two barley cultivars, Giza 124 (salt-tolerant) and Giza 119 (salt-sensitive).
2. Examine the effects of polyamine precursors, arginine, methionine, and ornithine, on the response of barley cultivars to salt stress.
3. Examine the effects of antioxidants specifically glutathione and ascorbic acid, on mitigating the harmful effects of salt stress on barley plants.
Grains of eight barley cultivars (Giza117, Giza119, Giza 121, Giza 123, Giza 124, Giza 125, Giza 126 and Giza 2000) obtained from the Egyptian Agricultural Research Center were tested for germination under different concentrations of NaCl (50 mM, 100 mM, 150 mM, and 200 mM). For the current study, Giza 124 was selected as the cultivar with the highest tolerance, while Giza 119 was identified as the most sensitive one.
Grains of two cultivars; salt-tolerant G124 and salt-sensitive G119 of barley (Hordeum vulgare L.) were germinated in plastic pots (10 cm diameter × 4 cm height each), filled with clay sandy soil (2:1). The pots were divided into five categories:
1. Presoaking Treatment:
Group 1: Grains were presoaked for 24 hours in distilled water.
Group 2: Grains were presoaked for 24 hours in an amino acid mixture (1 mM for each of Arginine, Methionine, and Ornithine).
Group 3: Grains were presoaked for 24 hours in a 0.1 mM solution of glutathione.
Group 4: Grains were presoaked for 24 hours in a 1 mM solution of ascorbic acid.
2. Salt Stress Treatment:
Group 5: One-week old seedlings were grown with 0.1 mM sodium chloride alone.
3. Antioxidants/amino acids Combined with Salt Treatment:
Group 6: One-week old seedlings were grown under the combined effect of 0.1 mM glutathione plus salt.
Group 7: One-week old seedlings were grown under the combined effect of 1 mM ascorbic acid plus salt.
Group 8: One-week old seedlings were grown under the combined effect of 1 mM amino acid mixture (Arginine, Methionine, and Ornithine) plus sal.
Growth criteria
After 21 d of exposure to each treatment, plants were harvested and separated into shoots and roots then shoot height, root depth, and leaf area were determined and weighed directly for the fresh weight (FW). Plant parts were dried in an air-driven oven at 80°C until constant weight for 48 hrs to determine the dry weight (DW).
Chlorophyll determination
A sample of fresh leaves (0.1 g) was homogenized in 5 ml 85% cold acetone at the seedling and pre-flowering stages, then centrifuged for 15 minutes at 3000 rpm and the pigment extracts were stored overnight in a refrigerator to prevent pigment degradation. The acetone extract was diluted to the appropriate volume, and the color intensities were measured at 663, 644, and 452.5 nm with a spectrophotometer to determine chlorophyll a, chlorophyll b, and carotenoids (Metzner et al., 1965). As pigments can be susceptible to degradation over time, measuring them promptly helps to ensure the accuracy of the results.
Measurement of photosynthetic efficiency
In the seedling and preflowering stages, photosynthetic efficiency (Fv/Fm) of dark-adapted leaves was measured with a portable pulse amplitude modulation (PAM) fluorometer (PerkinElmer, UK). Mature leaves, morphologically similar, were dark-adapted for 30 min then placed in the leaf clip, to maintain constant angles of incidence between the fibre-optic arm of the fluorometer and the leaf surface. The measurement of photosynthetic efficiency, on the adaxial leaf surface, was carried out as described by Gonçalves and Santos Júnior (2005). The leaf was initially exposed to the weak modulated measuring beam (< 0.1 umol/m2 per s), to estimate the initial fluorescence (F0), when the PS II reaction centers are open (oxidized). Thereafter, the leaf was then exposed to 800 ms saturation pulse of high-intensity (>10000 umol/m2 per s white light), to produce a transient closure (reduction) of the PS II reaction centers, at which point the maximum fluorescence (Fm), the variable fluorescence (Fv = Fm – F0), the maximum photochemical efficiency of PSII (Fv/Fm) and time of achieving maximum fluorescence yield (Tm) were obtained. Three replicates of each treatment were measured.
Determination of total soluble carbohydrates
The phenol sulfuric acid method has been used to estimate total soluble carbohydrates in the seedling, preflowering, and yield stages according to DuBois et al. (1956).
Borate buffer extraction of dried samples: One g of dry shoot or root was incubated with 5 ml borate buffer (pH8) for 24 h and then centrifuged for 15 min at 3000 rpm. The supernatant was completed to a known volume, then 0.1 ml of sample solution was transferred into a test tube and 1ml of phenol and 5 ml of sulfuric acid were added, then put in a water bath at 25 °C for 20 min. The color was read at 490 nm.
Determination of proline
The techniques according to Bates et al. (1973) and Zúñiga et al. (1989) were applied for the determination of proline in seedling and yield stages. One-half g of dry tissue was homogenized in 5 ml of 3% (w/v) sulfosalicylic acid and then filtered. To 2 ml of filtrate, 2 ml acid ninhydrin was added, followed by 2 ml glacial acetic acid then boiled for 60 min, and the reaction was terminated in an ice bath. The reaction mixture was extracted with toluene and mixed vigorously in a separating funnel for 20 sec. The chromophore containing toluene was aspirated from the aqueous phase and the absorbance was read at 520 nm, using toluene as a blank.
Assaying of antioxidant enzymes
Fresh leaves (0.5 g) of each barley cultivar were ground with 8 ml of 50 mM cold phosphate buffer (pH 7.8) and centrifuged at 4000 rpm for 20 min. The supernatant was used for the determination of the activities of antioxidant enzymes in the seedling stage.
Catalase (EC 1.11.1.6) was assayed (Kato & Shimizu, 1987) by measuring the initial rate of disappearance of H2O2. A sample of 3 ml of reaction mixture containing 0.1 M sodium phosphate buffer of pH 7.2, 11.8 mM H2O2, and 0.1 ml enzyme extract. The decrease in H2O2 was followed by a decline in the absorbance at 240 nm and the activity was calculated using the extinction coefficient (40 mM-1 cm-1 at 240 nm) for H2O2. The activity was expressed in units of μM of substrate converted per minute per one gram of fresh weight.
The activity of peroxidase (EC 1.11.1.7) was assessed using (Kato and Shimizu, 1987). The final assay volume was 3 ml, and the assay medium contained 0.1 M sodium phosphate buffer, pH 5.8, 7.2 mM guaiacol, 11.8 mM H2O2, and 0.1 ml enzyme extract. H2O2 was used to start the reaction, and a change in absorbance was detected at 470 nm.
Determination of lipid peroxidation
The lipid peroxidation was measured in the seedling stage by the amount of malonyl dialdehyde (MDA), as a product of peroxidation of unsaturated fatty acid (Linolenic acid, (18:3)). MDA concentration was estimated by the method of (Heath & Packer, 1968). A sample of 0.5 g fresh leaves was extracted in 10 ml 5% (w/v) trichloroacetic acid. The homogenate was then centrifuged at 4000 rpm for 10 min. The supernatant (2 ml) was mixed with 2ml of 0.67% (w/v) thiobarbituric acid, incubated at 100°C in a water bath for 20 min then cooled immediately. Absorbance was read at 532 nm and 600 nm. MDA concentration (μM/g FW) was calculated using the extinction coefficient 155Mm-1 cm-1.
Membrane leakage
Fresh leaves were taken and quickly cut into sections measuring about 0.5 cm in length for the assessment of electrolyte leakage in the seedling and pre-flowering stages. After being submerged for one hour in 20 ml of distilled water, one-half g of these leaf fragments was used to test the electrical conductivity in the leaking solution.
Mineral analysis of plant material
We used half a gram of oven-dried plant samples. The sample was digested using a semi-a micro Kjeldahl apparatus and 2 mL of concentrated perchloric acid and 4 mL of concentrated sulfuric acid. Digestion continued until a clear solution without charging was obtained, after which it was filtered using Whatman filter paper NO. 44 and completed up to a constant volume. Phosphorus was determined using the Molybdenum blue method and measuring optical density at 700 nm, nitrogen using the Indo-phenol blue method according to Tetlow & Wilson (1964) and measuring at 625 nm, and (K+) and (Na+) using the flame photometer (Allen et al., 1974). Protein nitrogen was calculated by the following equation:
Determination of biogenic amines by thin-layer chromatography (TLC)
Biogenic amines were extracted and determined in all tested samples according to Maijala and Eerola (1993).
The yield stage
Each barley cultivar was cultivated until the end of the growing season (starting from December 1st 2010 until the end of April 2011). The yield criteria were measured, including the weight of grains per plant, the weight of 100 grains, and the number of grains per plant.
All experimental determinations were replicated. The obtained data represented the mean values. Data obtained were analyzed statistically using Microsoft Excel software to determine the degree of significance between treatments for each cultivar. The one-way ANOVA method was applied for all data and differences between treatments were separated by the least significant differences (LSD) test at P<0.05 (Steel & Torrie, 1980).
1- Growth criteria
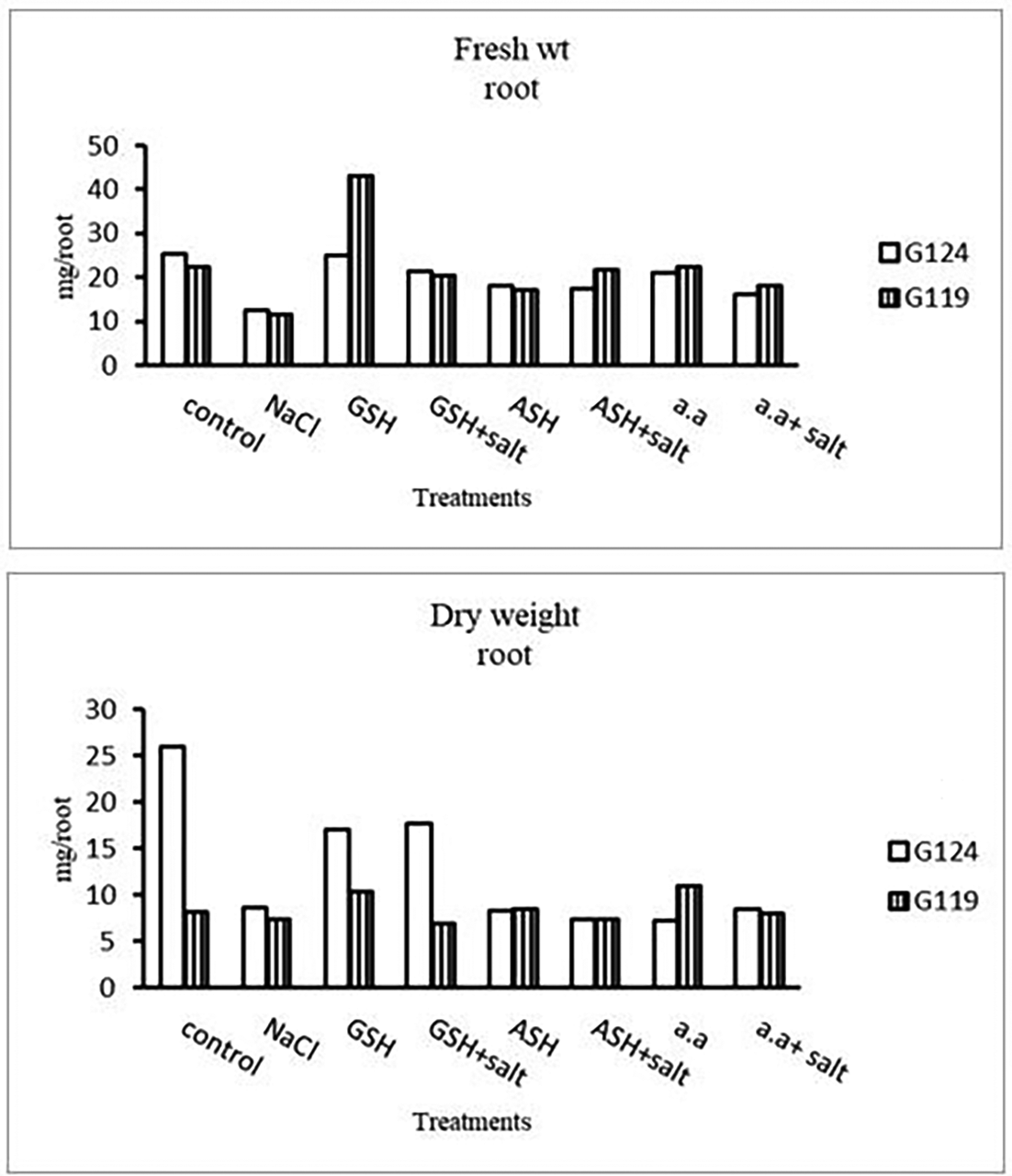
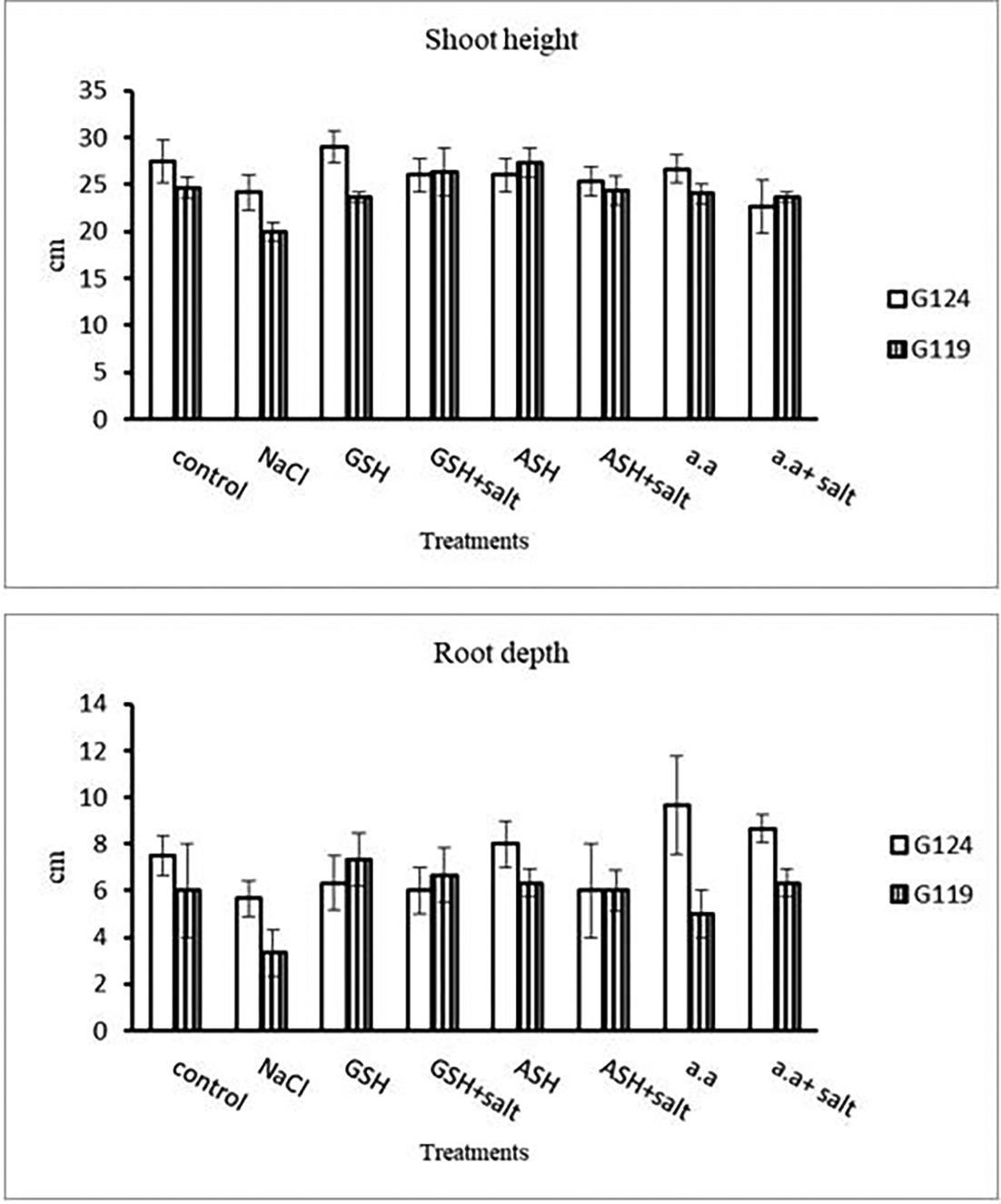
Grains of two barley cultivars, Giza 124, and Giza119 were germinated as described in the materials and methods section. Growth parameters (measured after 21 days), including fresh weight, dry weight, shoot height, root depth, and leaf area of barley seedlings were recorded as shown in Tables 1, 2, 3, and 4. The data showed that salinity stress caused a reduction in all studied growth parameters compared to the control. Also, it was observed that salinity resulted in a reduction in fresh and dry weights of both shoot and root of each cultivar compared to the control (Table 1 and Figure 1). Increases in the fresh and dry weights of each cultivar’s shoot and root following treatment with glutathione, ascorbic acid, or an amino acid combination offset the effects of salt. It is interesting to learn that, compared to other treatments, glutathione significantly increased the root dry weight of salt-affected Giza 124 (Table 2 and Figure 2). It was observed that shoot height and root depth in each barley cultivar decreased under salinity compared to control (Table 3 and Figure 3). However, shoot height increased in Giza 124 compared to Giza119 under the effect of glutathione, ascorbic acid or amino acid mixture. Moreover, root depth in Giza 124 reached its highest level under the effect of amino acid mixture compared to other treatments.
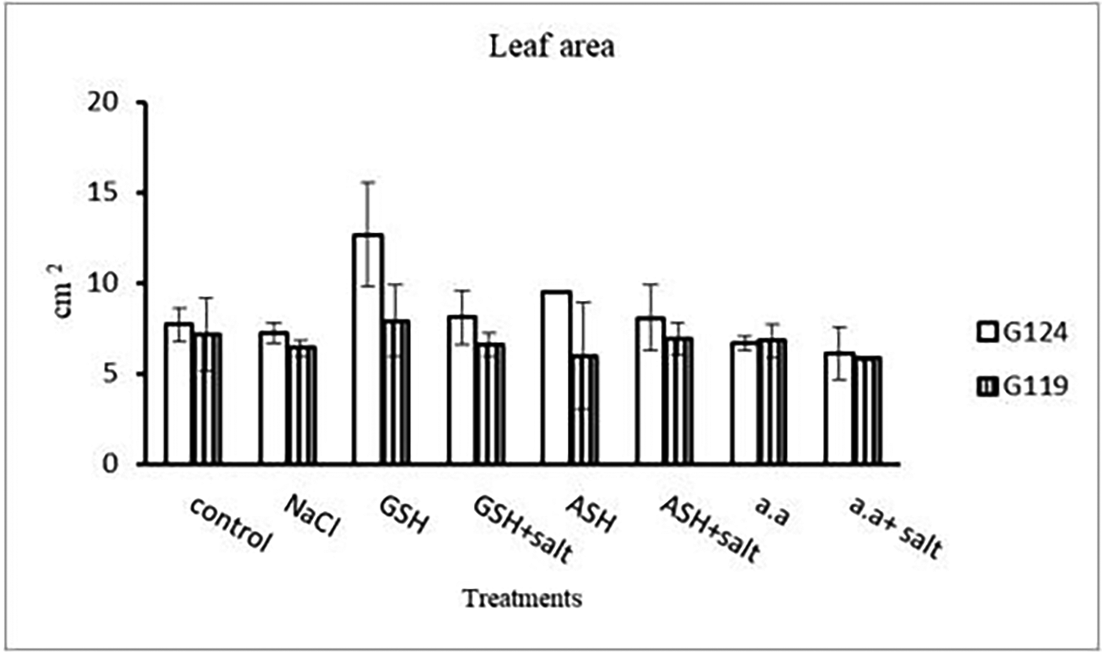
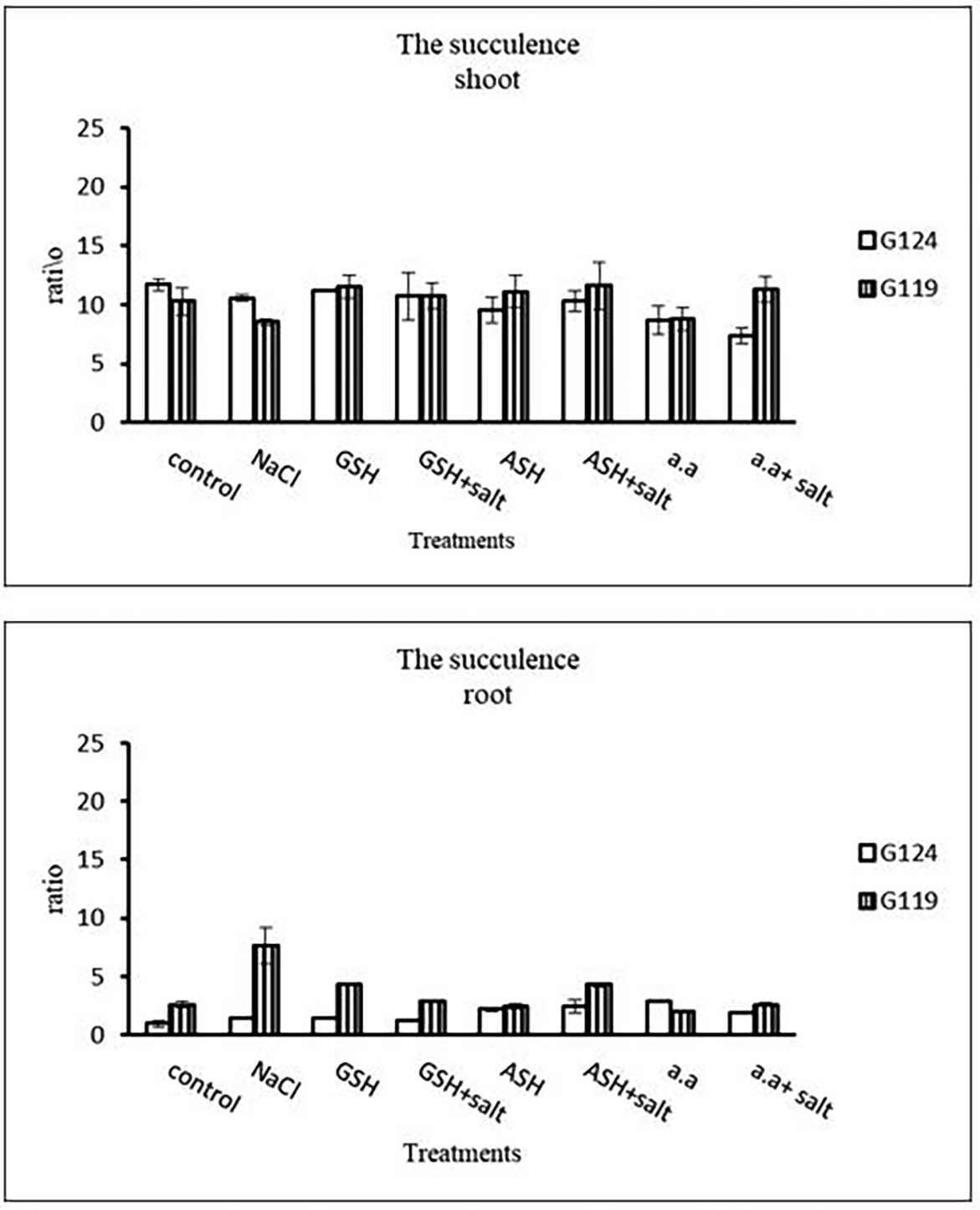
Results revealed that in Giza 124, the leaf area expanded significantly under the influence of glutathione but to a lesser extent under the influence of ascorbic acid as compared to control and other treatments (Table 4 and Figure 4), while glutathione showed a slight effect on Giza 119. The succulence ratio was decreased in the shoot and root of both cultivars with salinity but it increased in the root of Giza 119 under salinity and no variation was observed between other treatments (Table 5 and Figure 5).
| Treatments | Fresh weight | Dry weight | ||
|---|---|---|---|---|
| G124 | G119 | G124 | G119 | |
| Control | 746.3±18.6 | 599.6±47.4 | 63.7±04.2 | 58.3±2.8 |
| NaCl | 519.6±02.1 | 370.0±27.8 | 49.0±01.0 | 43.3±2.8 |
| Glutathione | 730.0±58.0 | 710.0±44.4 | 65.0±05.0 | 61.7±2.8 |
| Glutathione plus salt | 556.6±32.0 | 643.3±53.9 | 53.3±11.5 | 60.0±5.0 |
| Ascorbic acid | 540.0±61.0 | 663.3±27.5 | 56.3±1.53 | 60.0±5.0 |
| Ascorbic acid plus salt | 511.6±41.0 | 615.0±73.7 | 49.7±0.60 | 53.3±5.7 |
| Amino acid mixtures | 488.3±20.2 | 528.3±55.1 | 56.7±6.00 | 60.3±0.5 |
| Amino acids plus salt | 370.0±28.0 | 646.6±62.1 | 50.3±22.0 | 57.0±2.6 |
| F | 49.43 | 12.80 | 4.25 | 7.48 |
| P | ** | ** | ** | ** |
| LSD | 53.20 | 88.64 | 8.97 | 6.58 |

| Treatments | Fresh weight | Dry weight | ||
|---|---|---|---|---|
| G124 | G119 | G124 | G119 | |
| Control | 25.3±0.001 | 22.3±0.002 | 26.0±0.001 | 8.2±0.001 |
| NaCl | 12.3±0.001 | 11.3±0.001 | 9.0±0.001 | 7.4±0.001 |
| Glutathione | 25.0±0.001 | 43.0±0.001 | 17.0±0.001 | 10.3±0.001 |
| Glutathione plus salt | 21.3±0.001 | 20.3±0.001 | 17.7±0.001 | 7.0±0.001 |
| Ascorbic acid | 18.0±0.001 | 17.0±0.001 | 8.3±0.001 | 8.5±0.001 |
| Ascorbic acid plus salt | 17.3±0.001 | 22.0±0.001 | 7.5±0.001 | 7.3±0.001 |
| Amino acid mixtures | 21.0±0.001 | 22.3±0.001 | 7.3±0.001 | 11.0±0.001 |
| Amino acids plus salt | 16.0±0.001 | 18.0±0.001 | 8.6±0.001 | 8.0±0.001 |
| F | 8.25 | 288.2 | 193.01 | 21311.5 |
| P | ** | ** | ** | ** |
| LSD | 4.67 | 2.09 | 1.48 | 6.11 |
| Treatments | Shoot height | Root depth | ||
|---|---|---|---|---|
| G124 | G119 | G124 | G119 | |
| Control | 27.5 ±2.3 | 24.7 ± 1.1 | 7.5 ±0.9 | 6.0 ±2.0 |
| NaCl | 24.2 ±1.9 | 20.0 ± 1.0 | 5.7 ±0.8 | 3.3 ±1.0 |
| Glutathione | 29.0 ±1.7 | 23.7 ± 0.5 | 6.3 ±1.2 | 7.3 ±1.2 |
| Glutathione plus salt | 26.0 ±1.7 | 26.3 ± 2.5 | 6.0 ±1.0 | 6.7 ±1.2 |
| Ascorbic acid | 26.0 ±1.7 | 27.3 ± 1.5 | 8.0 ±1.0 | 6.3 ±0.6 |
| Ascorbic acid plus salt | 25.3±1.5 | 24.3 ± 1.5 | 6.0 ±2.0 | 6.0 ±0.9 |
| Amino acid mixtures | 26.7 ±1.5 | 24.0 ± 1.0 | 9.7 ±2.1 | 5.0 ±1.0 |
| Amino acids plus salt | 22.7 ±2.0 | 23.7± 0.5 | 8.7 ±0.6 | 6.3 ±0.6 |
| F | 2.96 | 7.50 | 3.72 | 2.161 |
| P | * | ** | * | ns |
| LSD | 3.38 | 2.37 | 2.37 | 1.94 |
| Treatments | Leaf area | |
|---|---|---|
| G124 | G119 | |
| Control | 7.7±0.9 | 7.1 ±1.99 |
| NaCl | 7.2±0.6 | 6.4±0.45 |
| Glutathione | 12.7 ±7.9 | 7.9±1.96 |
| Glutathione plus salt | 8.1±1.5 | 6.6±0.62 |
| Ascorbic acid | 9.5±0.1 | 5.9±2.96 |
| Ascorbic acid plus salt | 8.1±1.9 | 6.9±0.90 |
| Amino acid mixtures | 6.7±0.4 | 6.8±0.93 |
| Amino acids plus salt | 6.1±1.5 | 5.8±0.14 |
| F | 5.82 | 0.56 |
| P | ** | ns |
| LSD | 2.54 | 2.66 |
| Treatments | Succulence | |||
|---|---|---|---|---|
| Shoot | Root | |||
| G124 | G119 | G124 | G119 | |
| Control | 11.7±0.5 | 10.3±1.1 | 0.9±0.3 | 2.6±0.3 |
| NaCl | 10.6±0.3 | 8.5±0.2 | 1.4±0.1 | 7.6±1.5 |
| Glutathione | 11.2±0.1 | 11.5±1.0 | 1.5±0.1 | 4.3±0.1 |
| Glutathione plus salt | 10.7±1.9 | 10.8±1.1 | 1.2±0.1 | 2.9±0.1 |
| Ascorbic acid | 9.6 ±1.2 | 11.1±1.4 | 2.2±0.2 | 2.4±0.3 |
| Ascorbic acid plus salt | 10.3±0.9 | 11.6±2.0 | 2.4±0.6 | 4.3±0.2 |
| Amino acid mixtures | 8.7±1.2 | 8.8±0.9 | 2.9±0.1 | 22.3±0.6 |
| Amino acids plus salt | 7.4±0.6 | 11.3±1.1 | 1.9±0.1 | 2.6±0.1 |
| F | 5.94 | 2.99 | 20.30 | 31.38 |
| P | ** | * | ** | ** |
| LSD | 1.74 | 2.10 | 0.43 | 0.98 |
Statistical analysis showed that the effects of salinity, glutathione, ascorbic acid or amino acid mixture with or without salt were highly significant (P≤0.01) on each studied growth parameter compared to the control of each cultivar.
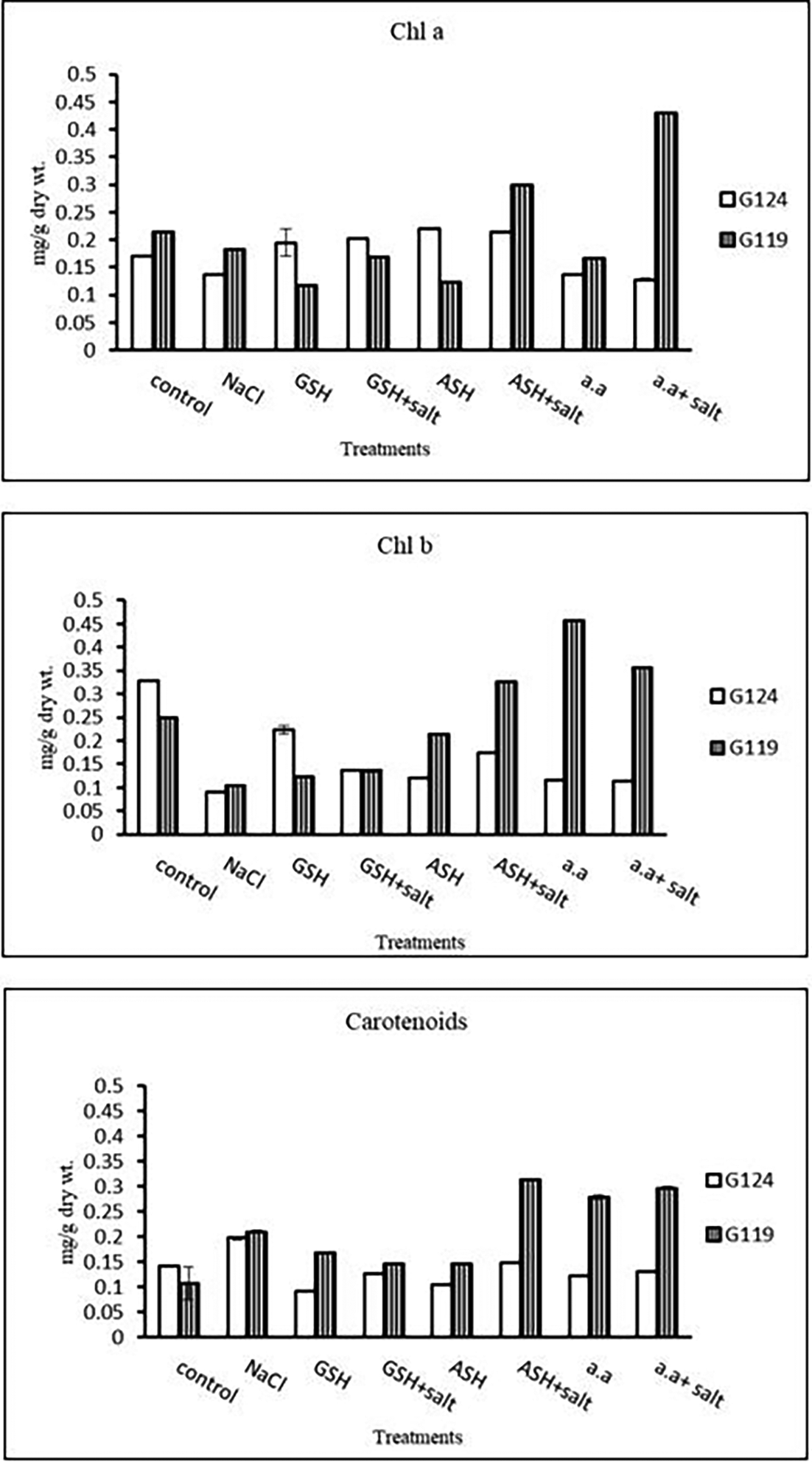
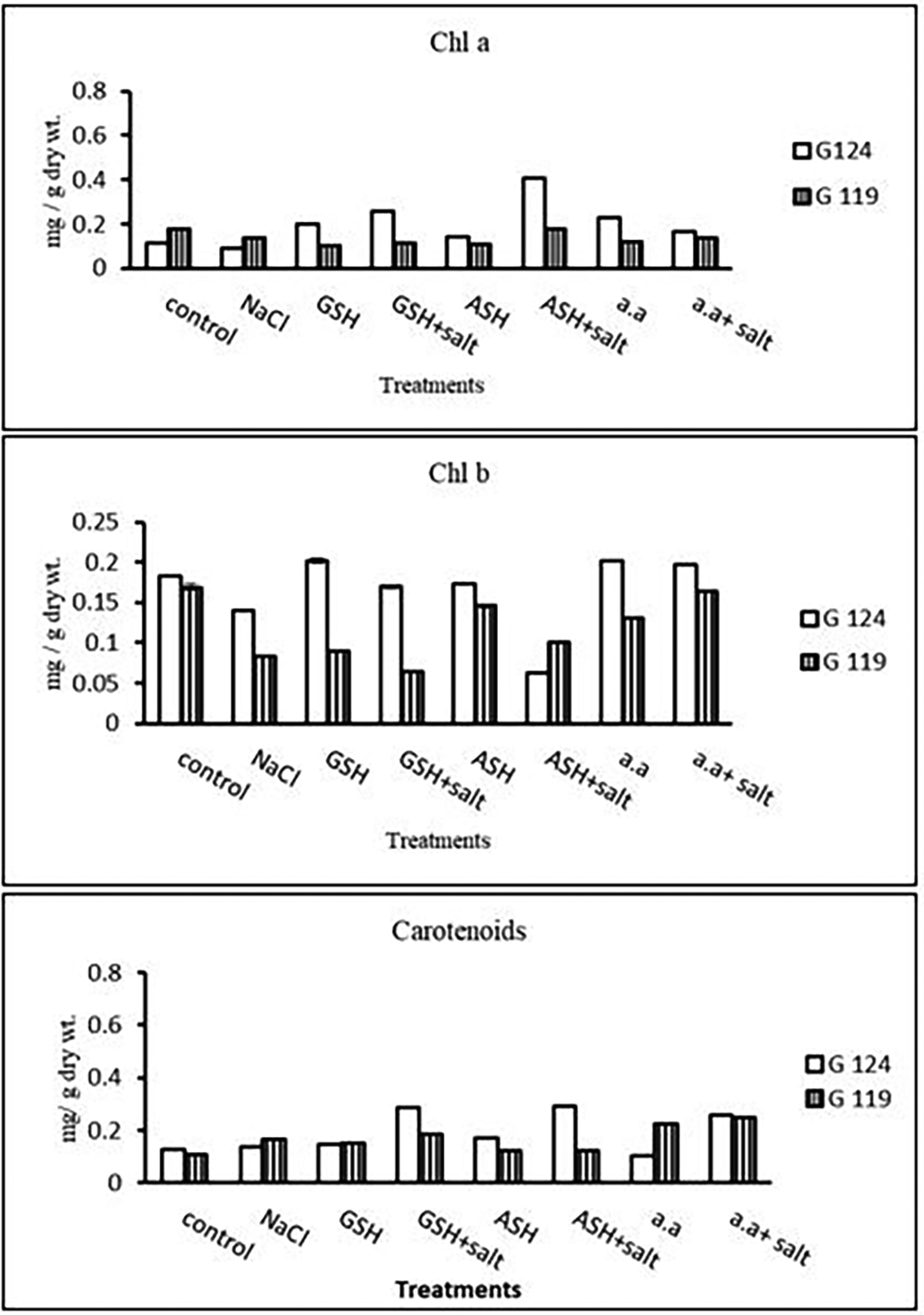
a) Chlorophyll contents: Regarding Giza 119, a remarkable rise of all determined pigments can be observed with the addition of either ascorbic acid or amino acid mixture to salt-stressed seedlings. However, the effect was more pronounced in chl b (Table 6 and Figure 6). Again, Giza 119 showed higher values of chl a, chl b, and carotenoids with the addition of ascorbic acid or amino acid mixture to salt-stressed seedlings and the difference were greater between the two cultivars. However, no remarkable effect of glutathione was observed with salt on most pigments.
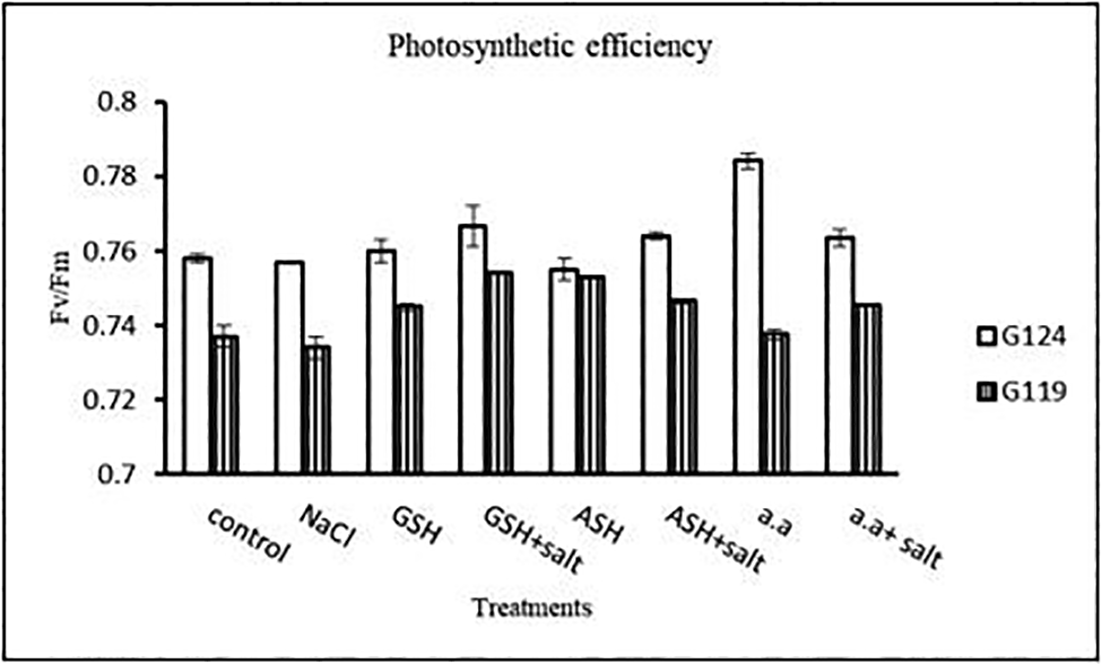
b) Photosynthetic efficiency: The addition of either glutathione, ascorbic acid or amino acid mixture with salt showed greater photosynthetic efficiency for each cultivar but the effect was greater with Giza 119 compared to Giza 124, which can be correlated with the pigment content (Table 7 and Figure 7).
| Treatments | Chlorophyll a | Chlorophyll b | Carotenoids | |||
|---|---|---|---|---|---|---|
| G124 | G119 | G 124 | G119 | G 124 | G119 | |
| Control | 0.17±0.0 | 0.21±0.0 | 0.33±0.0 | 0.25±0.0 | 0.14±0.0 | 0.11±0.0 |
| NaCl | 0.13±0.0 | 0.11±0.0 | 0.09±0.0 | 0.12±0.0 | 0.20±0.0 | 0.21±0.0 |
| Glutathione | 0.19±0.0 | 0.13±0.0 | 0.22±0.0 | 0.13±0.0 | 0.09±0.0 | 0.17±0.0 |
| Glutathione plus salt | 0.20±0.0 | 0.17±0.0 | 0.14±0.0 | 0.14±0.0 | 0.13±0.0 | 0.15±0.0 |
| Ascorbic acid | 0.22±0.0 | 0.18±0.0 | 0.12±0.0 | 0.19±0.0 | 0.10±0.0 | 0.14±0.0 |
| Ascorbic acid plus salt | 0.21±0.0 | 0.19±0.0 | 0.18±0.0 | 0.27±0.0 | 0.15±0.0 | 0.32±0.0 |
| Amino acid mixtures | 0.14±0.0 | 0.17±0.0 | 0.12±0.0 | 0.33±0.0 | 0.12±0.0 | 0.28±0.0 |
| Amino acids plus salt | 0.13±0.0 | 0.18±0.0 | 0.11±0.0 | 0.30±0.0 | 0.13±0.0 | 0.30±0.0 |
| F | 53.91 | 3284.81 | 1212.78 | 2082.19 | 2459.19 | 135.57 |
| P | ** | ** | ** | ** | ** | ** |
| LSD | 0.02 | 1.69 | 6.76 | 5.43 | 1.93 | 0.020 |
| Treatments | Photosynthetic efficiency | |
|---|---|---|
| G124 | G119 | |
| Control | 0.75±0.003 | 0.77±0.001 |
| NaCl | 0.74±0.002 | 0.76±0.001 |
| Glutathione | 0.76±0.002 | 0.75±0.001 |
| Glutathione plus salt | 0.77±0.001 | 0.72±0.002 |
| Ascorbic acid | 0.74±0.011 | 0.77±0.005 |
| Ascorbic acid plus salt | 0.75±0.001 | 0.78±0.001 |
| Amino acid mixtures | 0.76±0.003 | 0.78±0.001 |
| Amino acids plus salt | 0.73±0.003 | 0.78±0.002 |
| F | 9.06 | 1844.2 |
| P | ** | ** |
| LSD | 0.011 | 1.32 |
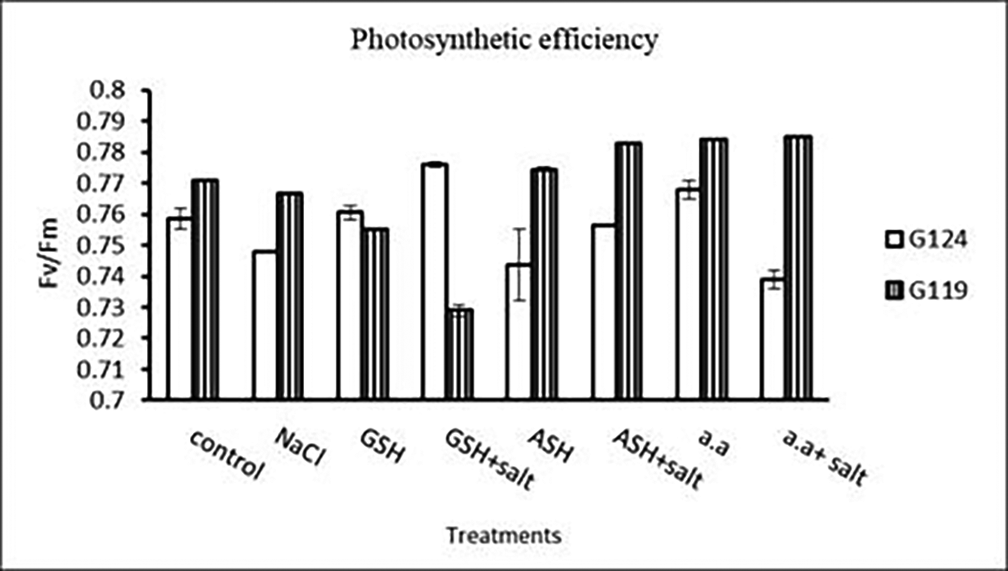
Statistical analysis showed that the effects of salinity, glutathione, ascorbic acid, or amino acid mixture with or without salt were highly significant (P≤0.01) on chlorophyll contents and photosynthetic efficiency of each cultivar.
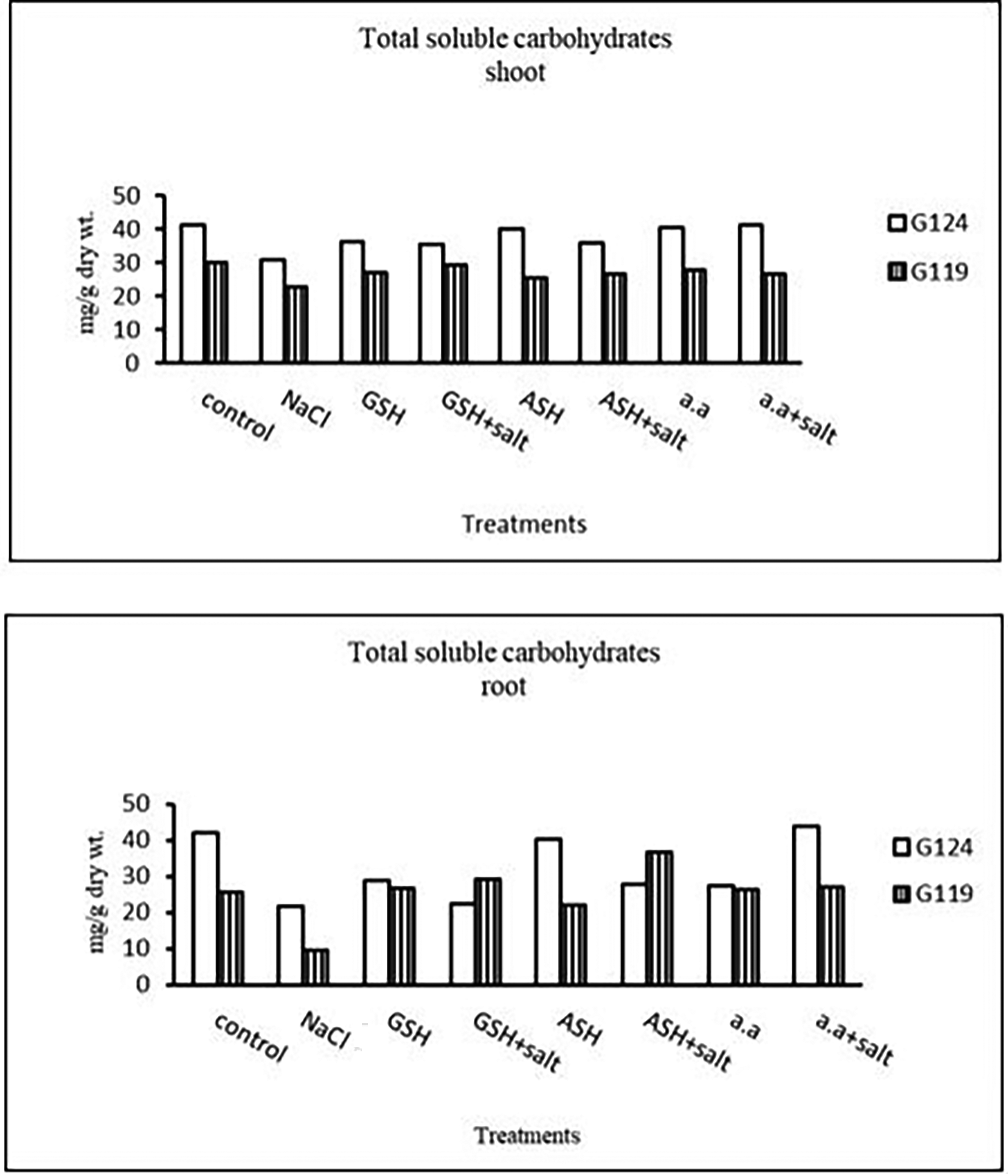
a) Total soluble carbohydrates: In each cultivar, there was a decrease in total soluble carbohydrates in shoot and root under salinity compared to the control (Table 8 and Figure 8). With the addition of glutathione, ascorbic acid, or amino acid mixture to salt total soluble carbohydrates increased.
b) Proline content: In both cultivars Salinity has resulted in an increase in proline content compared with the control and the effect was more pronounced in Giza 124 than Giza 119 (Table 9 and Figure 9). However, proline content decreased with glutathione, ascorbic acid, or amino acid mixture compared to control. Proline content decreased also with the addition of each of these compounds to salt compared with salt alone.
| Treatments | Shoot | Root | ||
|---|---|---|---|---|
| G124 | G119 | G124 | G119 | |
| Control | 41.3±0.0 | 30.0±0.1 | 41.9±0.0 | 25.6±0.2 |
| NaCl | 30.9 ±0.1 | 22.7±0.2 | 21.8±0.0 | 9.6±0.1 |
| Glutathione | 36.3±0.0 | 26.9±0.1 | 28.9±0.0 | 26.7±0.1 |
| Glutathione plus salt | 35.4±0.0 | 29.2±0.0 | 22.5±0.0 | 29.2±0.0 |
| Ascorbic acid | 40.0±0.1 | 25.5±0.1 | 40.1±0.0 | 22.1±0.1 |
| Ascorbic acid plus salt | 35.7±0.2 | 26.6±0.1 | 27.8±0.0 | 36.6±0.0 |
| Amino acid mixtures | 40.4±0.0 | 27.9±0.0 | 27.5±0.0 | 26.2±0.1 |
| Amino acids plus salt | 41.3±0.0 | 26.6±0.0 | 43.9±0.0 | 27.2±0.0 |
| F | 2347.45 | 1316.51 | 19213.68 | 21155.64 |
| P | ** | ** | ** | ** |
| LSD | 0.22 | 0.18 | 0.19 | 0.15 |
| Treatments | Proline | |
|---|---|---|
| G 124 | G119 | |
| Control | 38.4±0.1 | 8.7±0.1 |
| NaCl | 49.3±0.0 | 32.2±0.1 |
| Glutathione | 14.2±0.1 | 5.7±0.1 |
| Glutathione plus salt | 44.8±0.0 | 11.8±0.1 |
| Ascorbic acid | 12.7±0.4 | 6.3±0.1 |
| Ascorbic acid plus salt | 31.9±0.1 | 8.9±0.1 |
| Amino acid mixtures | 19.8±0.0 | 5.9±0.1 |
| Amino acids plus salt | 28.9±0.1 | 12.4±0.1 |
| F | 22416.1 | 39589.9 |
| P | ** | ** |
| LSD | 0.27 | 0.13 |
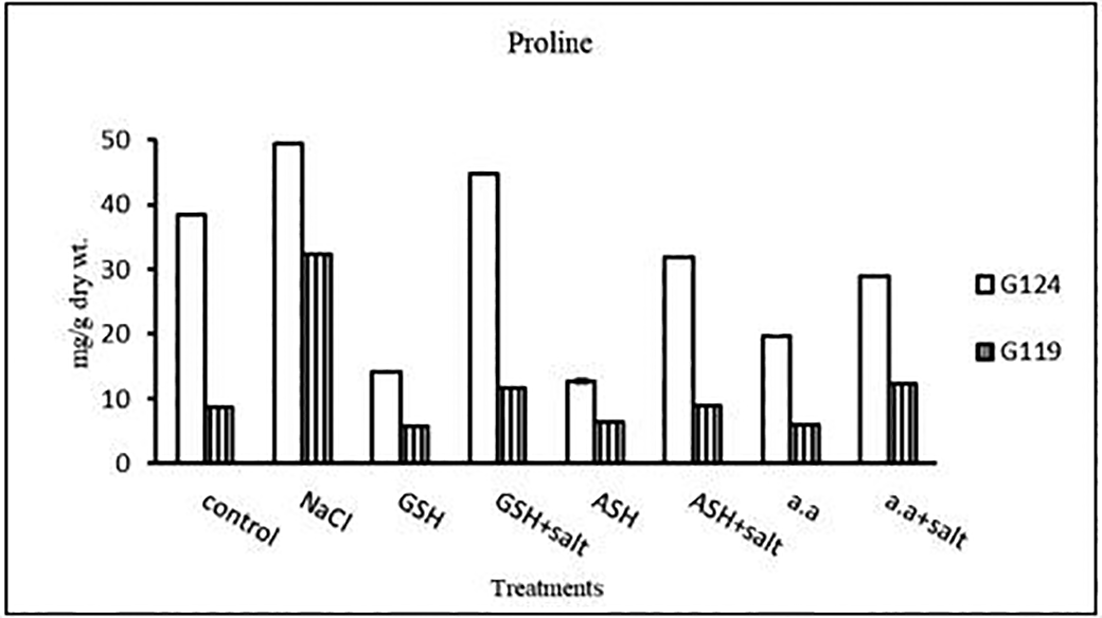
Statistical analysis showed that the effects of salinity, glutathione, ascorbic acid, or amino acid mixture with or without salt were highly significant (P≤0.01) the on total soluble carbohydrates and proline content of each cultivar.
4- Antioxidant enzymes: Results indicated that in each cultivar the activity of peroxidase and catalase increased under the effect of salinity compared to the control. The effect of salinity on peroxidase and catalase decreased with the addition of glutathione, ascorbic acid, or amino acid mixture (Table 10 and Figure 10).
5- Malondialdehyde content: In each cultivar, salinity showed an increase in malondialdehyde (MDA) content compared to the control. However, lipid peroxidation has decreased with glutathione, ascorbic acid, or amino acid mixture compared to the control. Giza 119 showed more MDA content compared to Giza 124 under salinity (Table 11 and Figure 11).
6- Membrane leakage: The results of both cultivars were more or less similar. However, salinity stress increased the membrane leakage of Giza 124 and Giza 119, while decreased by the addition of glutathione, ascorbic acid or amino acid mixture (Table 11 and Figure 11).
| Treatments | Peroxidase | Catalase | ||
|---|---|---|---|---|
| G124 | G119 | G124 | G119 | |
| Control | 5.7±1.2 | 4.6±0.6 | 0.08±0.0 | 0.07±0.0 |
| NaCl | 9.2±0.6 | 8.5±0.0 | 0.15±0.0 | 0.12±0.0 |
| Glutathione | 3.5±0.6 | 3.6±0.6 | 0.06±0.0 | 0.05±0.0 |
| Glutathione plus salt | 8.5±0.1 | 5.3±1.1 | 0.07±0.0 | 0.05±0.0 |
| Ascorbic acid | 2.1±1.1 | 1.8±0.6 | 0.05±0.0 | 0.05±0.0 |
| Ascorbic acid plus salt | 5.3±1.1 | 4.2±0.0 | 0.06±0.0 | 0.09±0.0 |
| Amino acid mixtures | 1.5±1.0 | 1.4±0.6 | 0.05±0.0 | 0.04±0.0 |
| Amino acids plus salt | 3.5±0.6 | 6.0±0.6 | 0.06±0.0 | 0.07±0.0 |
| F | 31.59 | 42.11 | 22.68 | 20.50 |
| P | ** | ** | ** | ** |
| LSD | 1.49 | 1.06 | 0.021 | 0.017 |

| Treatments | Malondialdehyde content | Membrane leakage | ||
|---|---|---|---|---|
| G124 | G119 | G124 | G119 | |
| Control | 132.5±1.2 | 28.7±3.7 | 54.2±0.2 | 38.8±0.4 |
| NaCl | 186.3±4.0 | 205.8±0.0 | 94.1±0.2 | 91.1±0.1 |
| Glutathione | 62.0±6.4 | 11.6±1.1 | 54.9±0.8 | 31.6±0.9 |
| Glutathione plus salt | 104.6±2.0 | 163.5±1.7 | 71.1±0.7 | 47.0±0.0 |
| Ascorbic acid | 31.0±8.9 | 13.9±3.4 | 62.3±0.6 | 38.3±0.5 |
| Ascorbic acid plus salt | 93.0±2.3 | 37.2±3.0 | 67.2±0.3 | 67.3±0.3 |
| Amino acid mixtures | 46.9±2.1 | 69.8±3.4 | 25.9±0.1 | 29.3±0.0 |
| Amino acids plus salt | 32.2±4.1 | 131.7±3.7 | 51.6±0.4 | 79.3±0.5 |
| F | 437.83 | 2959.37 | 4707.67 | 2977.99 |
| P | ** | ** | ** | ** |
| LSD | 7.78 | 5.03 | 0.84 | 1.28 |
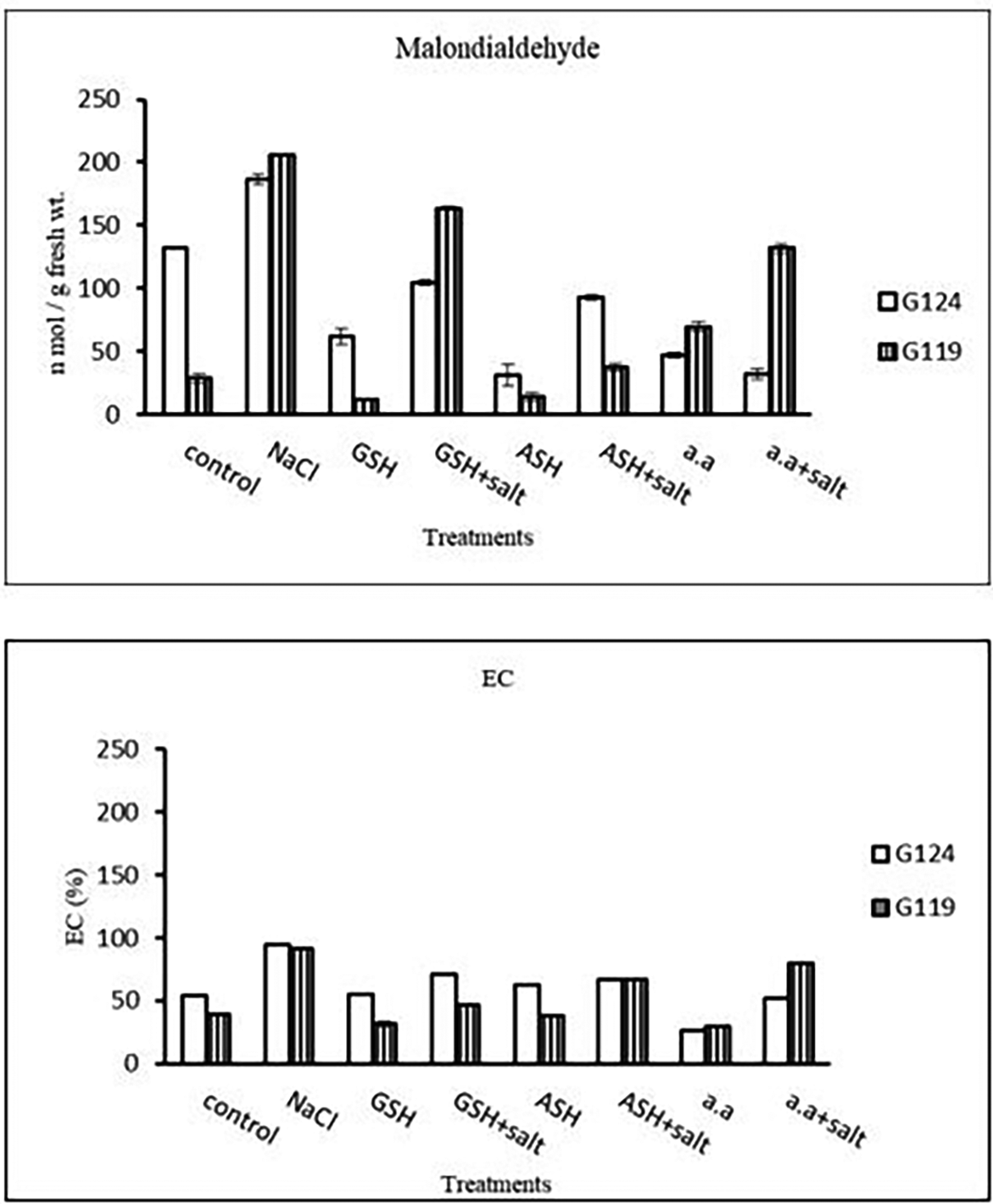
Statistical analysis showed that in each cultivar the effects of salinity, glutathione, ascorbic acid or amino acid mixture with or without salt were highly significant (P≤0.01) on the activity of each enzyme, MDA content and the membrane leakage of each cultivar.
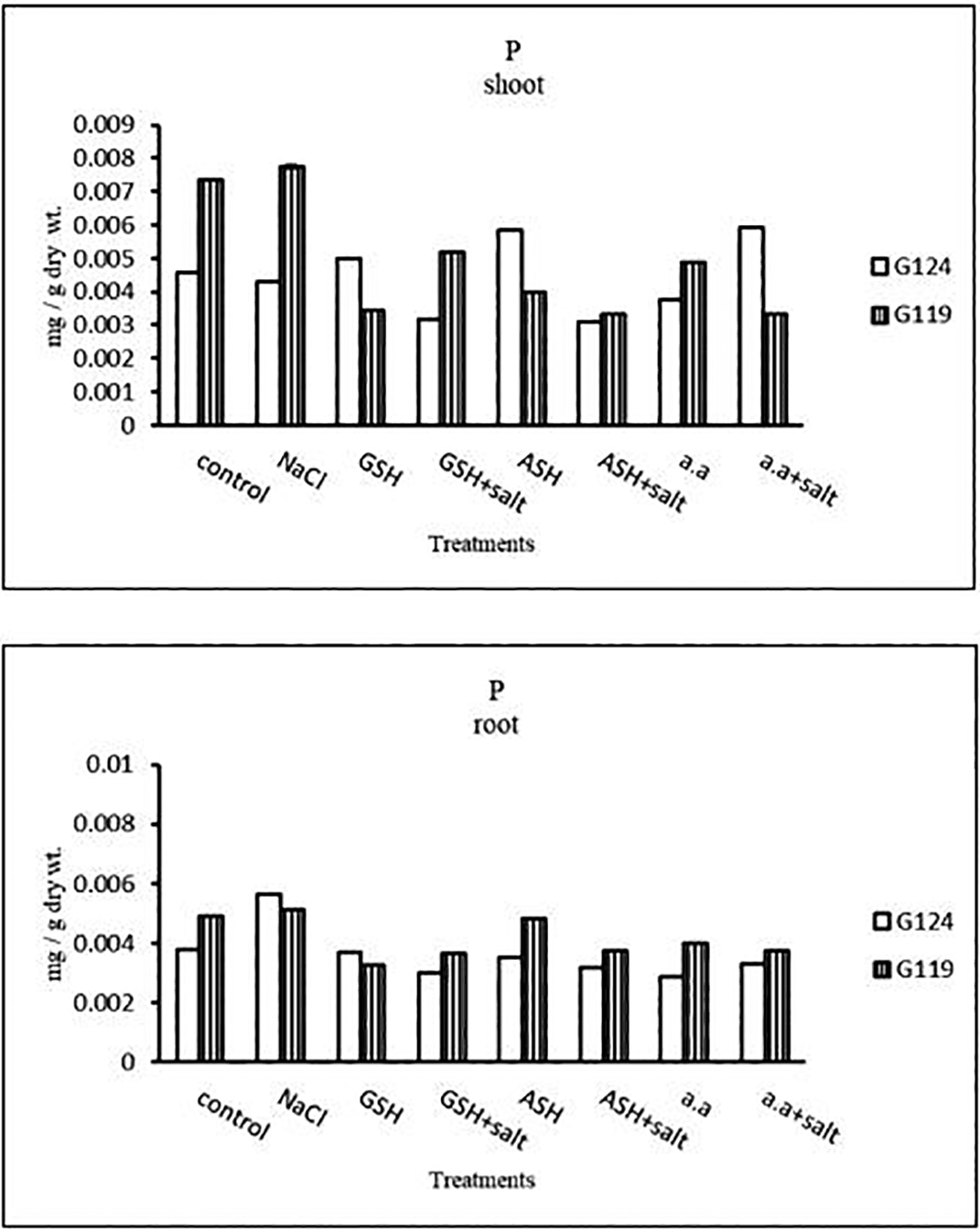
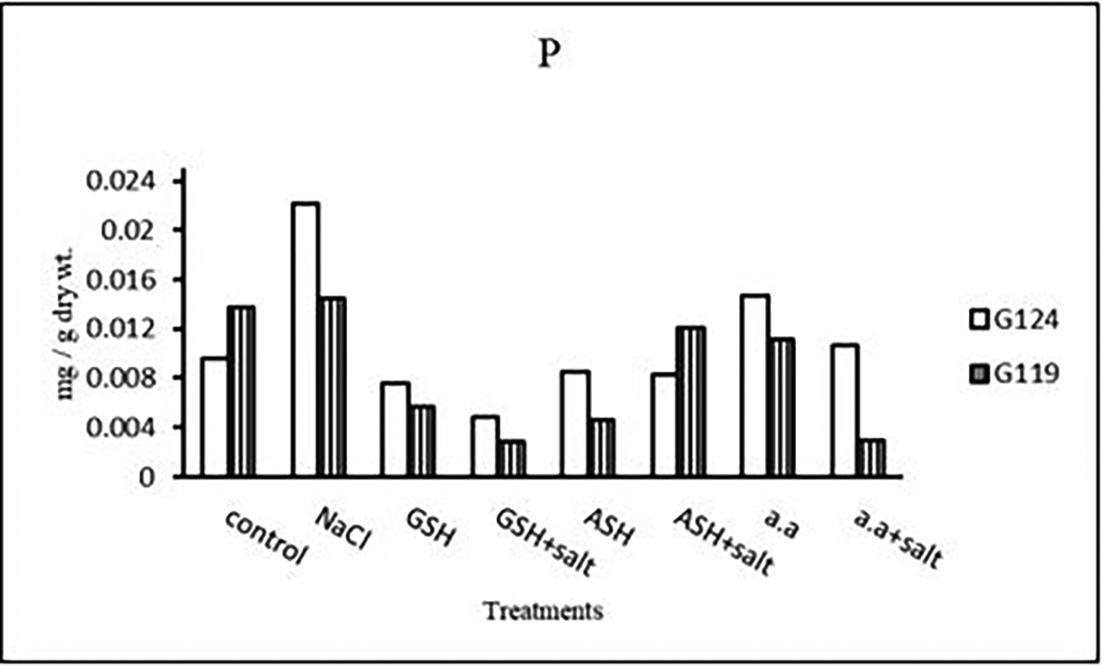
a) Phosphorus content: Salinity showed a slight increase in phosphorus content in the shoot and root of both cultivars (Table 12 and Figure 12). However, it increased in Giza 124 shoot under the effect of glutathione, ascorbic acid without salt or amino acid mixture plus salt. On the other hand, it decreased in Giza 124 root under the effect of glutathione, ascorbic acid, or amino acid mixture with or without salt compared to the control, while increased in Giza 119 root under the effect of ascorbic acid alone.
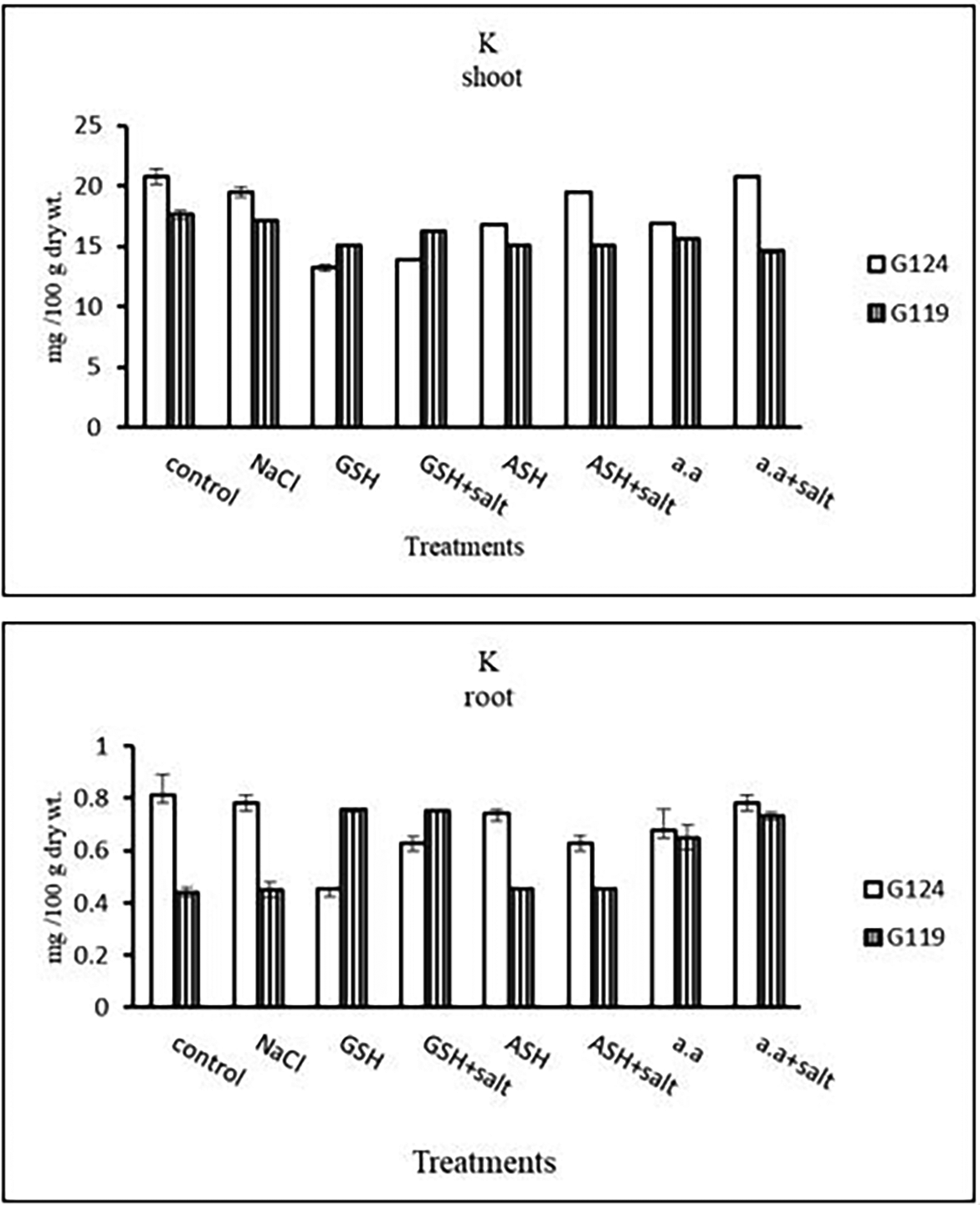
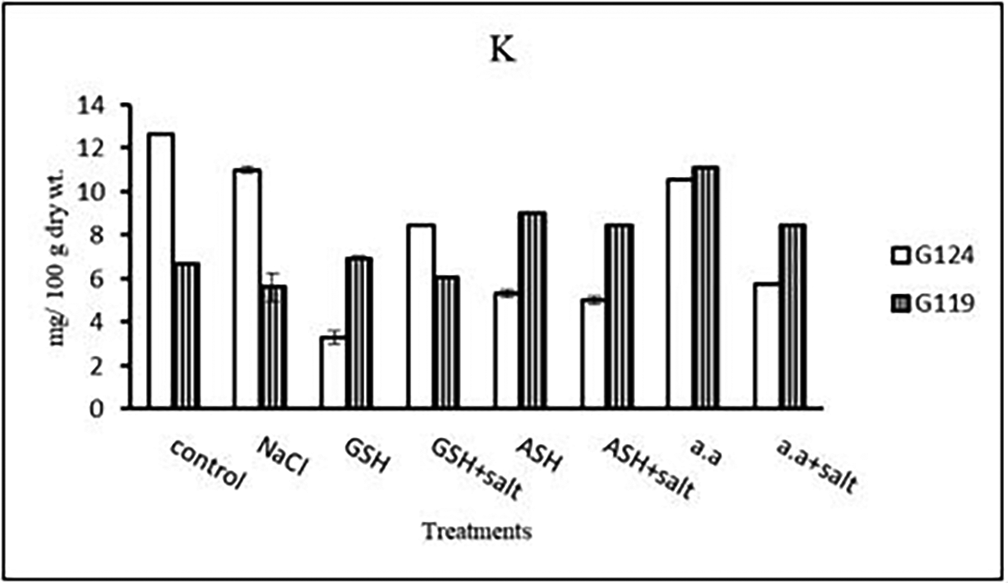
b) Potassium content: It can be noticed that potassium showed a decrease in the shoot and root of each cultivar, especially with salinity alone (Table 13 and Figure 13). However, Giza 124 shoot has experienced a rise in potassium content in most treatments and showed lower root potassium with salinity alone and ascorbic acid with or without salt or amino acids with salt compared to Giza 119.
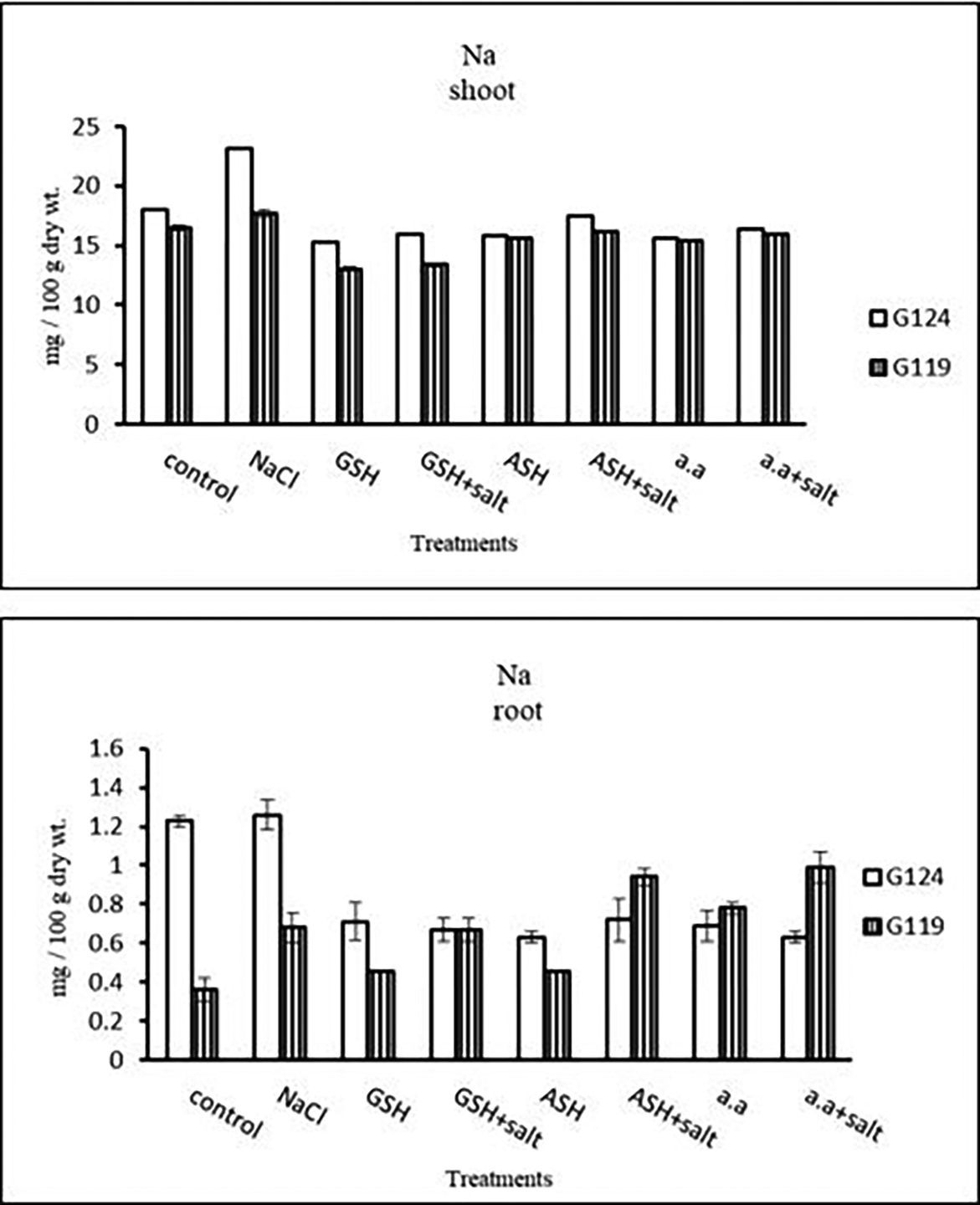
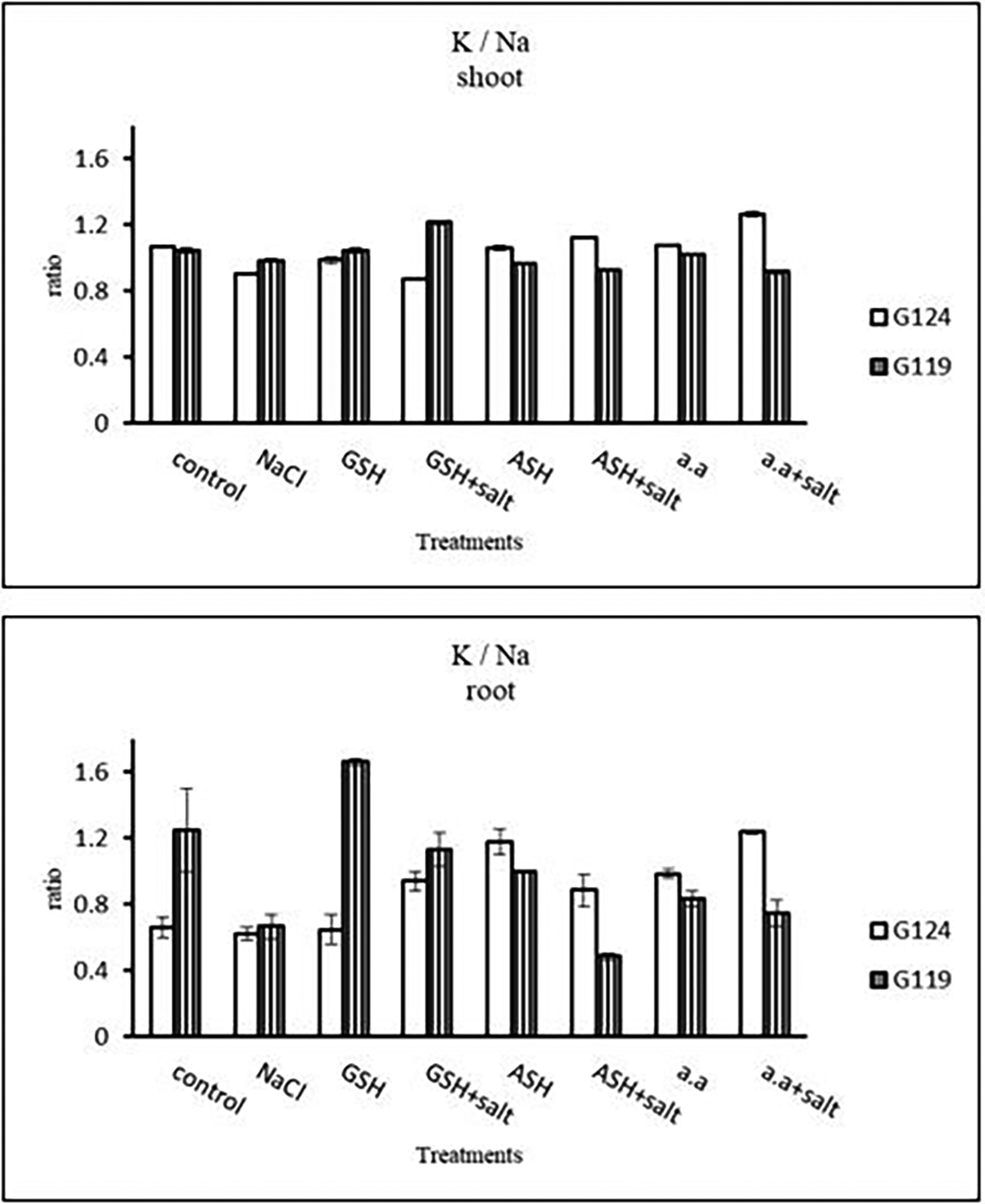
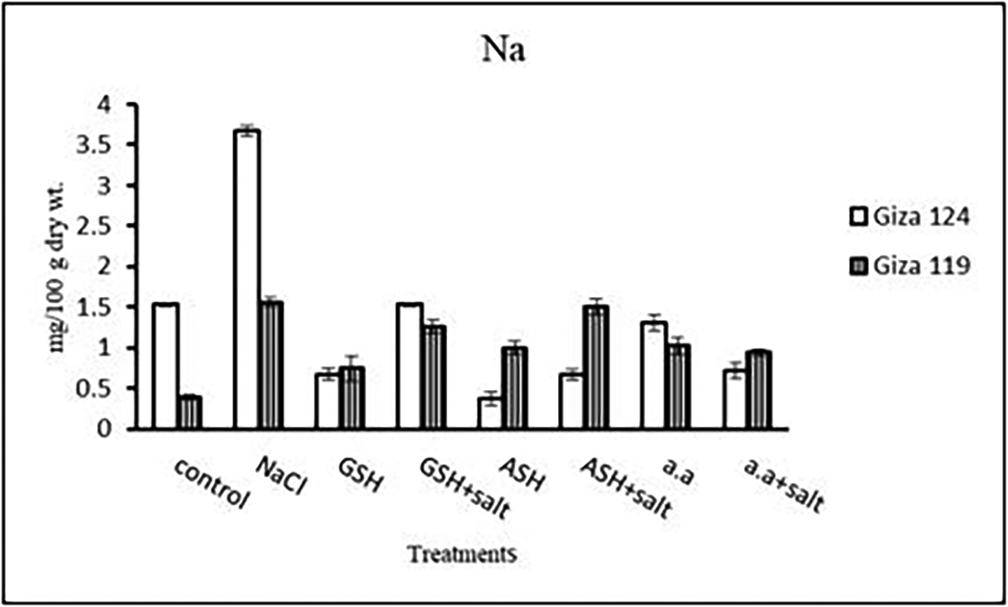
c) Sodium content: Results showed that sodium content increased under salinity in the shoot and root of both cultivars compared to the control (Table 14 and Figure 14). However it increased with glutathione, ascorbic acid, or amino acids with the salt in the root of Giza 119. Potassium/sodium ratio decreased with salt, while it has been increased with other treatments compared to the control (Table 16 and Figure 16).
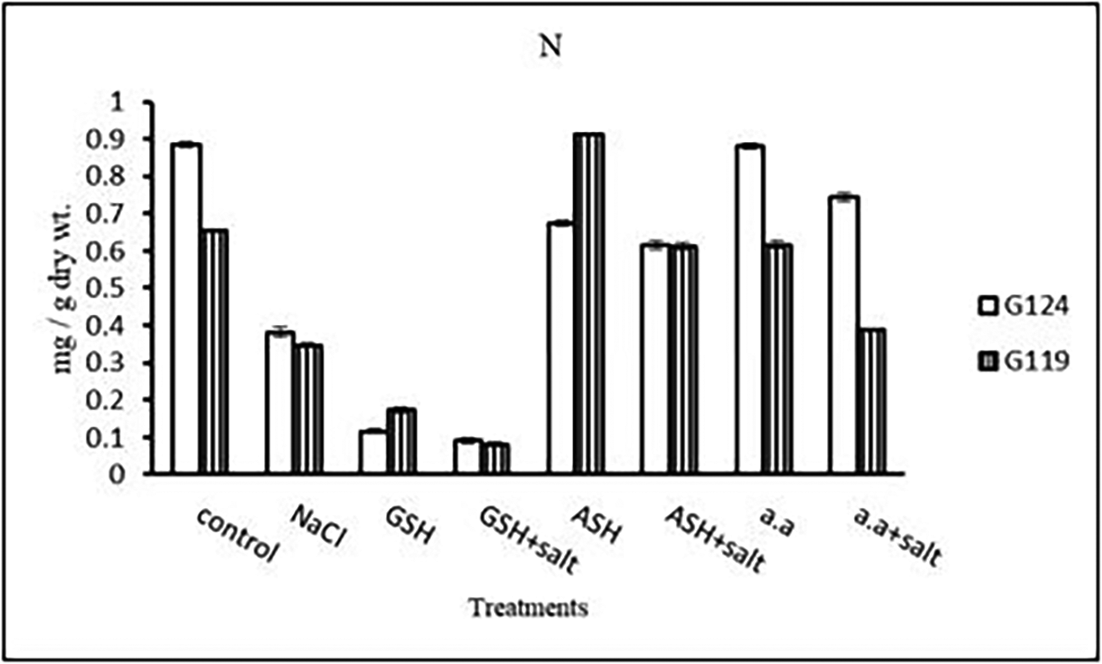
d) Nitrogen content: Results showed that nitrogen content increased in shoots of both Giza 124 and 119 under the effect of salinity compared to the control (Table 15 and Figure 15). However, it increased in the shoot and root of each cultivar under the effect of glutathione compared to other treatments.
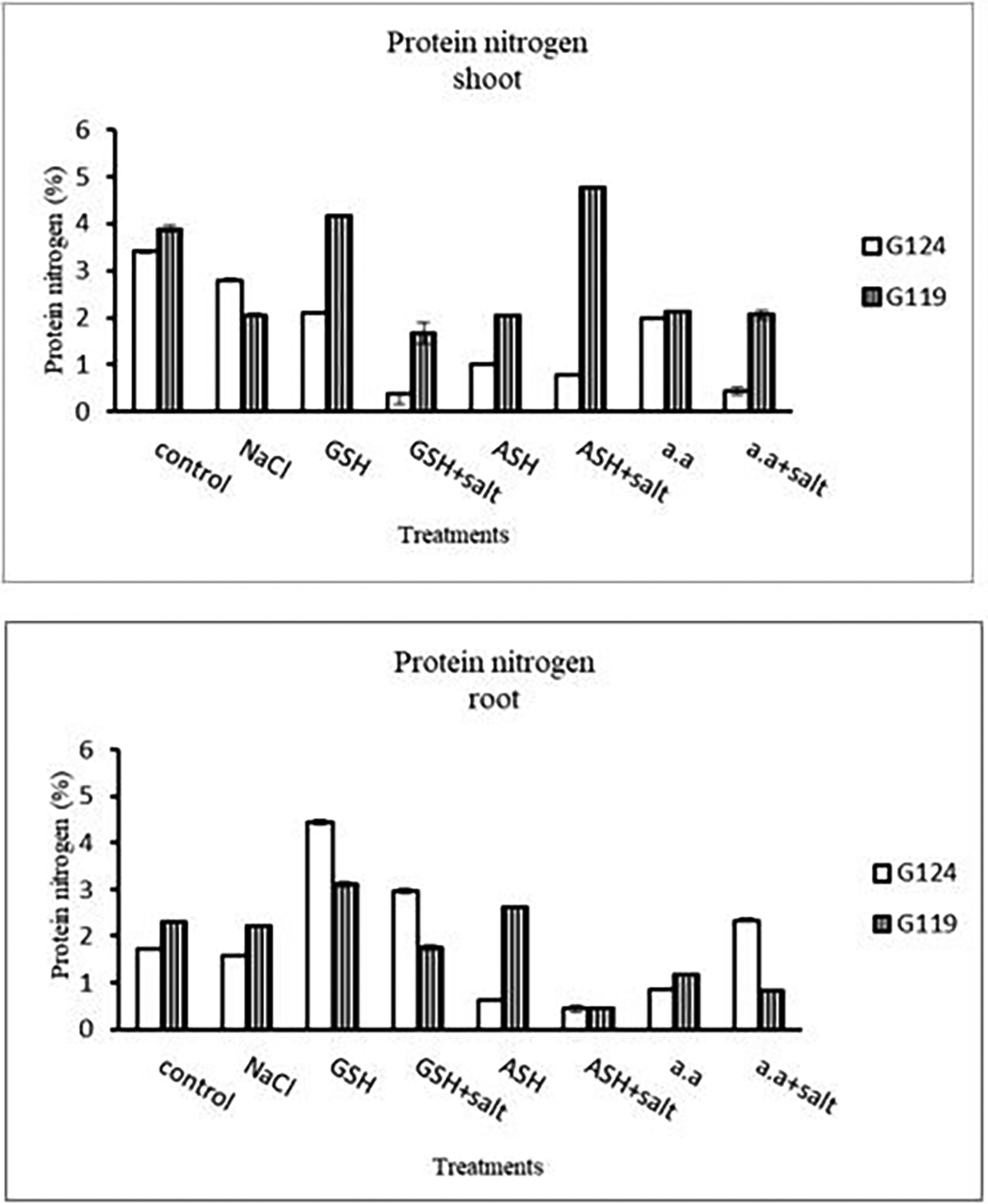
e) Protein nitrogen: Shoot protein nitrogen showed a decrease with salt in both cultivars and appreciably with glutathione, ascorbic acid, or amino acid plus salt in Giza 124 compared to other treatments (Table 17 and Figure 17). In both cultivars, root-protein nitrogen showed a remarkable increase with glutathione alone in Giza124 and Giza 119.
| Treatments | Phosphorus | |||
|---|---|---|---|---|
| Shoot | Root | |||
| G124 | G119 | G124 | G119 | |
| Control | 0.004±0.0 | 0.008±0.0 | 0.006±0.0 | 0.005±0.0 |
| NaCl | 0.005±0.0 | 0.007±0.0 | 0.004±0.0 | 0.004±0.0 |
| Glutathione | 0.005±0.0 | 0.003±0.0 | 0.004±0.0 | 0.003±0.0 |
| Glutathione plus salt | 0.003±0.0 | 0.005±0.0 | 0.003±0.0 | 0.004±0.0 |
| Ascorbic acid | 0.006±0.0 | 0.004±0.0 | 0.004±0.0 | 0.005±0.0 |
| Ascorbic acid plus salt | 0.003±0.0 | 0.003±0.0 | 0.003±0.0 | 0.004±0.0 |
| Amino acid mixtures | 0.004±0.0 | 0.005±0.0 | 0.003±0.0 | 0.004±0.0 |
| Amino acids plus salt | 0.006±0.0 | 0.003±0.0 | 0.003±0.0 | 0.004±0.0 |
| F | 4294.7 | 7480.7 | 1105.7 | 1725.6 |
| P | ** | ** | ** | ** |
| LSD | 4.99 | 6.12 | 7.90 | 4.99 |
| Treatments | Potassium | |||
|---|---|---|---|---|
| Shoot | Root | |||
| G124 | G119 | G124 | G119 | |
| Control | 20.8±0.7 | 17.6±0.3 | 0.8±0.1 | 0.4±0.0 |
| NaCl | 19.5±0.5 | 17.1±0.0 | 0.7±0.0 | 0.4±0.0 |
| Glutathione | 13.2±0.3 | 15.1±0.2 | 0.4±0.0 | 0.7±0.0 |
| Glutathione plus salt | 13.8±0.0 | 16.8±0.1 | 0.6±0.0 | 0.7±0.0 |
| Ascorbic acid | 16.8±0.0 | 15.1±0.1 | 0.7±0.0 | 0.4±0.0 |
| Ascorbic acid plus salt | 19.5±0.0 | 15.1±0.2 | 0.6±0.0 | 0.4±0.0 |
| Amino acid mixtures | 16.9±0.1 | 15.7±0.1 | 0.7±0.1 | 0.6±0.1 |
| Amino acids plus salt | 20.8±0.1 | 14.6±0.2 | 0.7±0.0 | 0.7±0.0 |
| F | 21.9 | 177.0 | 22.2 | 147.9 |
| P | ** | ** | ** | ** |
| LSD | 0.07 | 0.03 | 0.25 | 0.12 |
| Treatments | Sodium | |||
|---|---|---|---|---|
| Shoot | Root | |||
| G124 | G119 | G124 | G119 | |
| Control | 18.0±0.0 | 16.4±0.2 | 1.23±0.03 | 0.36±0.03 |
| NaCl | 23.3±0.1 | 17.7±0.3 | 1.26±0.07 | 0.68±0.01 |
| Glutathione | 15.3±0.1 | 13.0±0.1 | 0.71±0.09 | 0.45±0.06 |
| Glutathione plus salt | 15.9±0.1 | 13.4±0.1 | 0.67±0.06 | 0.67±0.01 |
| Ascorbic acid | 15.8±0.0 | 15.6±0.1 | 0.63±0.03 | 0.46±0.01 |
| Ascorbic acid plus salt | 17.4±0.1 | 16.2±0.0 | 0.72±0.10 | 0.94±0.04 |
| Amino acid mixtures | 15.6±0.1 | 15.3±0.1 | 0.69±0.07 | 0.78±0.03 |
| Amino acids plus salt | 16.4±0.0 | 15.9±0.1 | 0.63±0.03 | 0.99±0.07 |
| F | 834.3 | 292.9 | 42.2 | 87.7 |
| P | ** | ** | ** | ** |
| LSD | 0.26 | 0.27 | 0.12 | 0.07 |
| Treatments | Nitrogen | |||
|---|---|---|---|---|
| shoot | root | |||
| G124 | G119 | G124 | G119 | |
| Control | 0.55±0.0 | 0.33±0.0 | 0.25±0.0 | 0.36±0.0 |
| NaCl | 0.02±0.0 | 0.62±0.0 | 0.01±0.0 | 0.36±0.0 |
| Glutathione | 0.14±0.0 | 0.67±0.0 | 0.71±0.0 | 0.49±0.0 |
| Glutathione plus salt | 0.06±0.0 | 0.27±0.0 | 0.47±0.0 | 0.28±0.0 |
| Ascorbic acid | 0.16±0.0 | 0.22±0.0 | 0.09±0.0 | 0.41±0.0 |
| Ascorbic acid plus salt | 0.12±0.0 | 0.76±0.0 | 0.01±0.0 | 0.07±0.0 |
| Amino acid mixtures | 0.06±0.0 | 0.34±0.0 | 0.14±0.0 | 0.18±0.0 |
| Amino acids plus salt | 0.31±0.0 | 0.33±0.0 | 0.37±0.0 | 0.13±0.0 |
| F | 988.9 | 548.6 | 4247.3 | 1231.5 |
| P | ** | ** | ** | ** |
| LSD | 0.02 | 0.02 | 0.01 | 0.01 |

| Treatments | K/Na ratio | |||
|---|---|---|---|---|
| shoot | root | |||
| G124 | G119 | G124 | G119 | |
| Control | 1.06±0.00 | 1.04±0.01 | 0.66±0.06 | 1.25±0.25 |
| NaCl | 0.91±0.01 | 0.99±0.01 | 0.62±0.04 | 0.67±0.07 |
| Glutathione | 0.99±0.02 | 1.04±0.01 | 0.65±0.09 | 1.66±0.02 |
| Glutathione plus salt | 0.87±0.00 | 1.22±0.01 | 0.94±0.05 | 1.13±0.10 |
| Ascorbic acid | 1.06±0.01 | 0.96±0.01 | 1.18±0.08 | 0.99±0.00 |
| Ascorbic acid plus salt | 1.12±0.00 | 0.93±0.01 | 0.88±0.10 | 0.48±0.01 |
| Amino acid mixtures | 1.10±0.00 | 1.02±0.00 | 0.99±0.02 | 0.83±0.04 |
| Amino acids plus salt | 1.27±0.01 | 0.92±0.01 | 1.24±0.01 | 0.74±0.07 |
| F | 286.6 | 201.4 | 38.1 | 34.2 |
| P | ** | ** | ** | ** |
| LSD | 0.02 | 0.01 | 0.11 | 0.19 |
| Treatments | Protein nitrogen | |||
|---|---|---|---|---|
| Shoot | Root | |||
| G124 | G119 | G124 | G119 | |
| Control | 3.4±0.03 | 3.9±0.06 | 1.6±0.03 | 2.3±0.01 |
| NaCl | 2.8±0.04 | 2.1±0.04 | 1.7±0.02 | 2.3±0.03 |
| Glutathione | 2.1±0.03 | 4.2±0.00 | 4.4±0.05 | 3.1±0.05 |
| Glutathione plus salt | 0.4±0.03 | 1.7±0.22 | 2.9±0.05 | 1.8±0.04 |
| Ascorbic acid | 1.0±0.03 | 2.0±0.02 | 0.6±0.01 | 2.6±0.01 |
| Ascorbic acid plus salt | 0.8±0.03 | 4.8±0.03 | 0.4±0.07 | 0.4±0.01 |
| Amino acid mixtures | 2.0±0.00 | 2.1±0.03 | 0.9±0.01 | 1.2±0.01 |
| Amino acids plus salt | 0.4±0.01 | 2.1±0.10 | 2.3±0.03 | 0.8±0.01 |
| F | 2519.3 | 495.5 | 2850.1 | 2727.2 |
| P | ** | ** | ** | ** |
| LSD | 0.06 | 0.16 | 0.07 | 0.05 |
Statistical analysis showed that the effects of salinity, glutathione, ascorbic acid, or amino acid mixture with or without salt were highly significant (P≤0.01) in phosphorus, potassium, sodium, K+/Na+ ratio, nitrogen, and protein nitrogen.
8- Polyamines (PAs) content: Determination of polyamine content under salt stress indicated an increase in spermidine and spermine in Giza 119 compared to Giza 124 while putrescine increased in both cultivars with salt compared to control (Table 18). Addition of glutathione with salt caused an increase in putrescine and spermidine of Giza 124 and ascorbic acid with salt resulted in higher putrescine content in each cultivar with an increase in spermidine of Giza 119 only. Also, addition of amino acid mixture with salt showed an increase in putrescine and spermidine in each cultivar. The amount of spermine was so small to be detected with other treatments.
1- Photosynthetic pigments: In Giza 124 Chl a and Chl b were most dominant in all treatments. A remarkable rise has been observed in Chl b with glutathione, ascorbic acid, or amino acid mixture alone in G124 (Table 19 and Figure 18). Regarding carotenoids, Giza 124 has higher levels with glutathione plus salt or ascorbic acid plus salt compared to Giza 119.
2- Photosynthetic efficiency: Photosynthetic efficiency decreased with salt in each cultivar. In general, Giza 124 showed slightly higher photosynthetic efficiency compared to the Giza119 cultivar, particularly with glutathione or amino acid mixture with or without salt (Table 20 and Figure 19).
3- Total Soluble Carbohydrates: At the pre-flowering stage, salinity showed a decrease in total soluble carbohydrates in each cultivar compared to the control (Table 21 and Figure 20). By the addition of either glutathione, ascorbic acid or amino acid mixture an increase was detected in total soluble carbohydrates of each cultivar.
4- Membrane Leakage: Results showed that membrane leakage was increased under the effect of salinity in each cultivar compared to the control (Table 22 and Figure 21). Glutathione plus salt showed a decrease in membrane leakage in G124 while Giza119 showed a decrease with amino acid plus salt compared to other treatments.
| Treatments | Chlorophyll a | Chlorophyll b | Carotenoids | |||
|---|---|---|---|---|---|---|
| G124 | G119 | G 124 | G119 | G 124 | G119 | |
| Control | 0.09±0.0 | 0.13±0.0 | 0.14±0.0 | 0.18±0.0 | 0.12±0.0 | 0.16±0.0 |
| NaCl | 0.11±0.0 | 0.18±0.0 | 0.18±0.0 | 0.07±0.0 | 0.13±0.0 | 0.11±0.0 |
| Glutathione | 0.17±0.0 | 0.10±0.0 | 0.89±0.0 | 0.09±0.0 | 0.15±0.0 | 0.15±0.0 |
| Glutathione plus salt | 0.26±0.0 | 0.11±0.0 | 0.17±0.0 | 0.06±0.0 | 0.28±0.0 | 0.18±0.0 |
| Ascorbic acid | 0.14±0.0 | 0.11±0.0 | 0.23±0.0 | 0.12±0.0 | 0.17±0.0 | 0.12±0.0 |
| Ascorbic acid plus salt | 0.39±0.0 | 0.17±0.0 | 0.43±0.0 | 0.29±0.0 | 0.29±0.0 | 0.12±0.0 |
| Amino acid mixtures | 0.16±0.0 | 0.12±0.0 | 0.30±0.0 | 0.13±0.0 | 0.10±0.0 | 0.23±0.0 |
| Amino acids plus salt | 0.23±0.0 | 0.13±0.0 | 0.17±0.0 | 0.35±0.0 | 0.26±0.0 | 0.25±0.0 |
| F | 1680.25 | 9871.8 | 22345.1 | 1636.1 | 4255.8 | 22517.2 |
| P | ** | ** | ** | ** | ** | ** |
| LSD | 7.06 | 3.53 | 4.99 | 6.11 | 3.53 | 9.99 |
| Treatments | Photosynthetic efficiency | |
|---|---|---|
| G124 | G119 | |
| Control | 0.76±0.0 | 0.74±0.0 |
| NaCl | 0.75±0.0 | 0.73±0.0 |
| Glutathione | 0.76±0.0 | 0.75±0.0 |
| Glutathione plus salt | 0.77±0.0 | 0.75±0.0 |
| Ascorbic acid | 0.76±0.0 | 0.75±0.0 |
| Ascorbic acid plus salt | 0.76±0.0 | 0.75±0.0 |
| Amino acid mixtures | 0.78±0.0 | 0.74±0.0 |
| Amino acids plus salt | 0.76±0.0 | 0.75±0.0 |
| F | 22.66 | 45.14 |
| P | ** | ** |
| LSD | 6.11 | 3.53 |
| Treatments | Total soluble carbohydrates | |
|---|---|---|
| G124 | G119 | |
| Control | 19.8±0.0 | 44.4±0.2 |
| NaCl | 4.5±0.2 | 26.7±0.2 |
| Glutathione | 20.6±0.1 | 27.6±0.2 |
| Glutathione plus salt | 12.7±0.2 | 30.1±0.0 |
| Ascorbic acid | 14.1±0.1 | 25.8±0.2 |
| Ascorbic acid plus salt | 12.4±0.1 | 34.7±0.1 |
| Amino acid mixtures | 13.8±0.1 | 30.8±0.0 |
| Amino acids plus salt | 9.7±0.0 | 35.4±0.0 |
| F | 4435.7 | 9681.6 |
| P | ** | ** |
| LSD | 0.34 | 0.18 |

| Treatments | Membrane leakage | |
|---|---|---|
| G124 | G119 | |
| Control | 49.1±0.2 | 41.2±0.8 |
| NaCl | 96.2±0.6 | 91.2±0.4 |
| Glutathione | 90.9±0.6 | 41.8±0.1 |
| Glutathione plus salt | 50.7±0.3 | 81.6±0.2 |
| Ascorbic acid | 42.5±0.4 | 35.8±0.1 |
| Ascorbic acid plus salt | 91.6±0.2 | 86.0±1.8 |
| Amino acid mixtures | 69.2±0.3 | 63.5±0.3 |
| Amino acids plus salt | 94.3±1.1 | 73.7±0.1 |
| F | 11085.3 | 6402.9 |
| P | ** | ** |
| LSD | 0.65 | 0.83 |
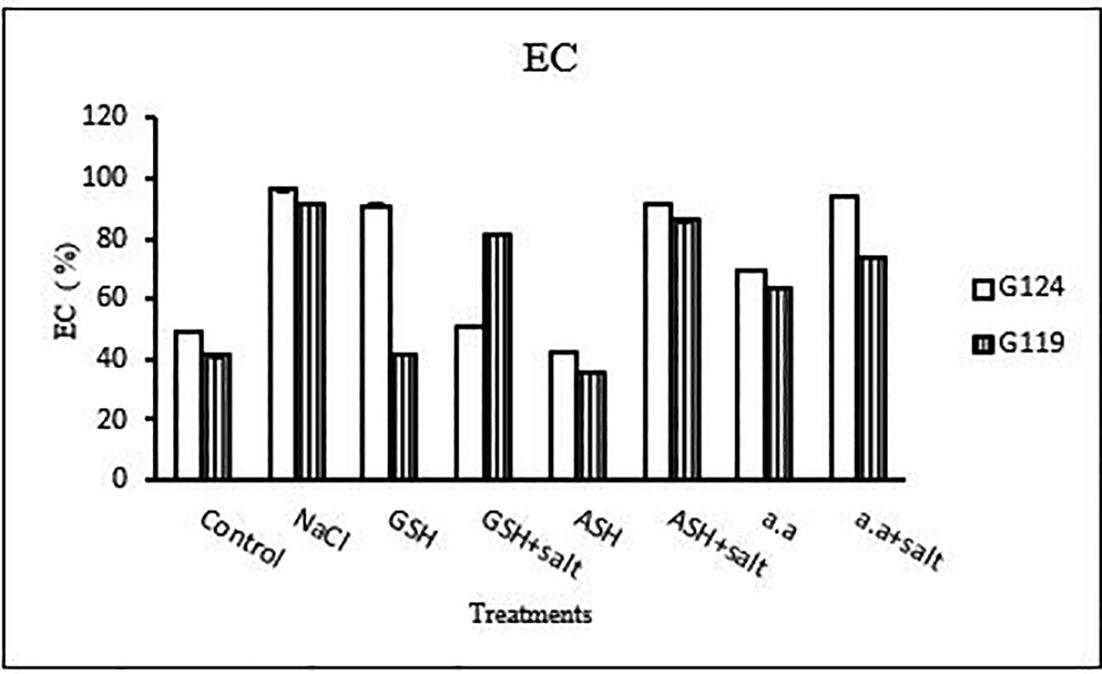
Statistical analysis showed that the effects of salinity, glutathione, ascorbic acid or amino acid mixture with or without salt were highly significant (P≤0.01) on photosynthetic pigments, photosynthetic efficiency, total soluble carbohydrates, and membrane leakage.
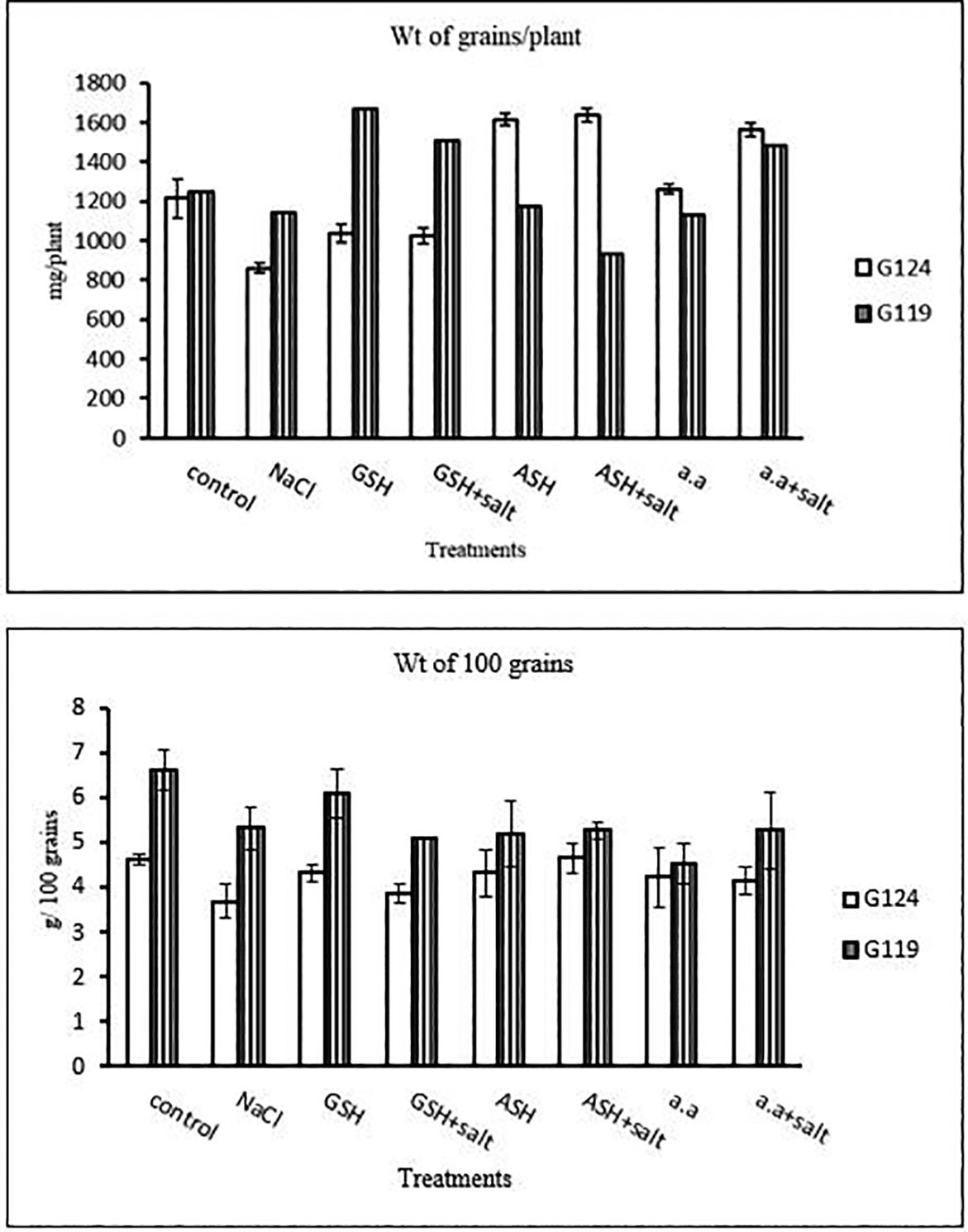
1- Yield criteria: In both cultivars weight of grains per plant and weight of 100 grains has decreased under the effect of salinity compared to the control (Table 23 and Figure 22). Grains per plant were increased in Giza 124 with either ascorbic acid or amino acid mixture and in Giza 119 with glutathione alone or plus salt and amino acid plus salt in Giza 119.
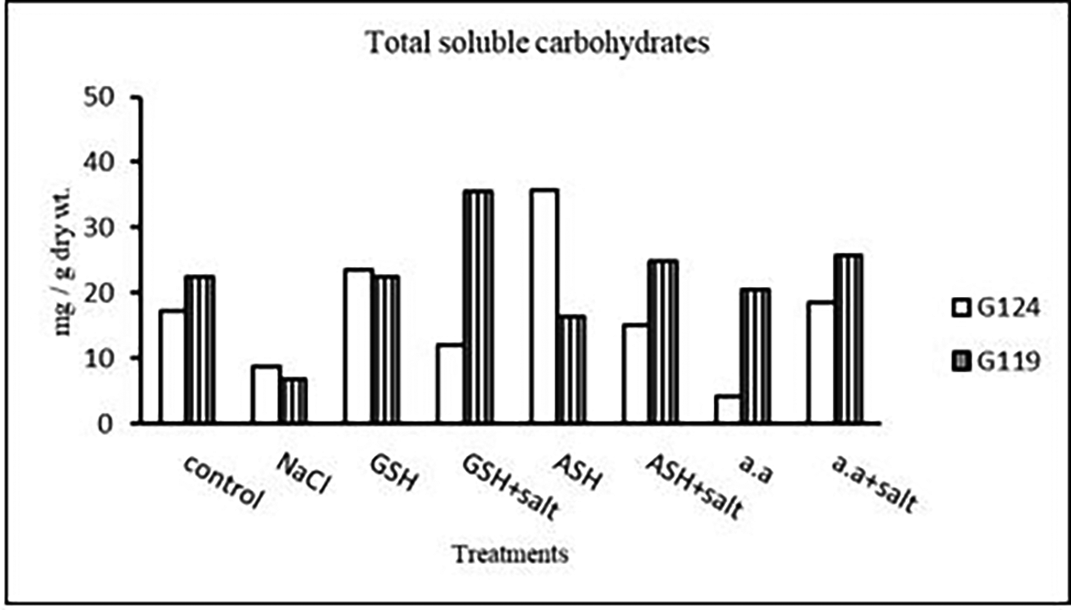
2- Total soluble carbohydrates: It can be noticed (Table 24 and Figure 23) that salinity brought about a drop in total soluble carbohydrates in each cultivar but increased in both with glutathione alone. The addition of glutathione, ascorbic acid or amino acid mixture to salt has resulted in a rise of T.S.C in Giza 119-seeds compared to that of Giza 124.
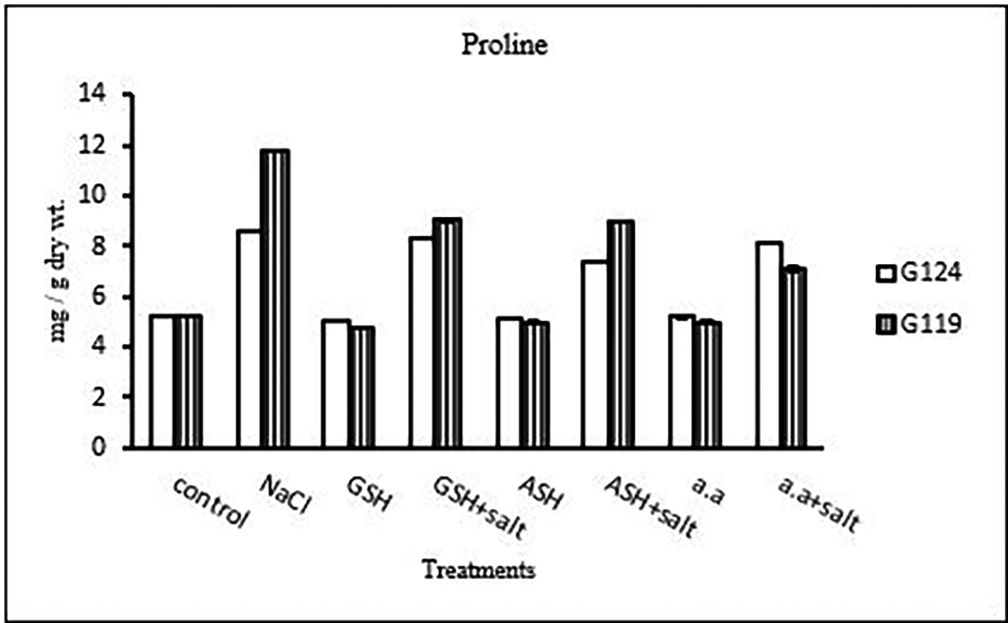
3- Proline content: In both cultivars, a specific increase in grain proline has been seen when sodium chloride, glutathione, ascorbic acid, or an amino acid mixture are combined with salt in comparison to control (Table 25, and Figure 24). According to statistical analysis, each cultivar’s proline content, total soluble carbohydrates, and yield criteria were all significantly affected by salinity, glutathione, ascorbic acid, or amino acid mixtures with or without salt (P≤0.01).
4- Mineral analysis
a) Phosphorous content: In each cultivar, phosphorus content was increased under salinity compared to the control (Table 26 and Figure 25). Phosphorus content increased in Giza 124 with amino acid mixture alone compared to 119, while increased in Giza 119 with ascorbic acid plus salt.
b) Potassium content: In both cultivars, potassium content showed a low level under salinity compared to control (Table 27 and Figure 26). On the other hand, potassium content increased in Giza 119 with glutathione, ascorbic acid or amino acid mixture with or without salt.
c) Sodium content: An apparent rise has been detected in Na content of Giza 124 and Giza 119. Other treatments showed a decrease in sodium content in each cultivar (Table 28 and Figure 27). K+/Na+ ratio decreased under salinity in each cultivar, while it increased with ascorbic acid alone in G124 and with glutathione, ascorbic acid or amino acid mixture without salt in G119 (Table 30 and Figure 29).
d) Nitrogen content: In both cultivars, nitrogen content showed a remarkable reduction with salinity (Table 29 and Figure 28). However, the highest content was observed with the amino acid mixture in Giza 124 and ascorbic acid in Giza 119, while the lowest one was observed in both cultivars with glutathione plus salt.
e) Protein nitrogen: In comparison to other treatments, protein nitrogen decreased with salt in both cultivars and noticeably with glutathione in two cultivars, either with or without salt (Table 31 and Figure 30). With ascorbic acid or an amino acid and salt, protein nitrogen increased in both cultivars.
| Treatments | Weight of grains/plant | Weight of 100 grians | ||
|---|---|---|---|---|
| G124 | G119 | G124 | G119 | |
| Control | 1213.3±101.2 | 1248.3±5.77 | 4.62±0.1 | 6.62±0.4 |
| NaCl | 860.0±26.5 | 1141.7±7.63 | 3.70±0.4 | 5.33± 0.4 |
| Glutathione | 1038.3±46.5 | 1668.3±88.1 | 4.33±0.2 | 6.12± 0.4 |
| Glutathione plus salt | 1026.7±40.1 | 1508.3±7.63 | 3.87±0.2 | 5.12±0.0 |
| Ascorbic acid | 1613.3±30.6 | 1175.0±26.5 | 4.33± 0.5 | 5.20± 0.7 |
| Ascorbic acid plus salt | 1636.7± 32.1 | 931.7±40.1 | 4.66± 0.3 | 5.29±0.2 |
| Amino acid mixtures | 1261.7±25.2 | 1130.0±15.0 | 4.25± 0.7 | 4.54±0.4 |
| Amino acids plus salt | 1561.7±33.3 | 1485.0±70.0 | 4.17±0.3 | 5.29±0.9 |
| F | 115.72 | 93.78 | 2.36 | 4.50 |
| P | ** | ** | ns | ** |
| LSD | 83.03 | 75.88 | 0.64 | 0.91 |
| Treatments | Total soluble carbohydrates | |
|---|---|---|
| G124 | G119 | |
| Control | 17.3±0.1 | 22.5±0.1 |
| NaCl | 8.6±0.0 | 6.7±0.0 |
| Glutathione | 23.6±0.0 | 22.4±0.0 |
| Glutathione plus salt | 11.9±0.1 | 35.4±0.0 |
| Ascorbic acid | 35.7±0.3 | 16.4±0.0 |
| Ascorbic acid plus salt | 15.1±0.0 | 24.7±0.1 |
| Amino acid mixtures | 4.0±0.0 | 20.4±0.0 |
| Amino acids plus salt | 18.5±0.1 | 25.8±0.1 |
| F | 21566.9 | 86611.5 |
| P | ** | ** |
| LSD | 0.20 | 0.08 |
| Treatments | Proline | |
|---|---|---|
| G124 | G119 | |
| Control | 5.2±0.1 | 5.2±0.1 |
| NaCl | 8.6±0.0 | 11.8±0.0 |
| Glutathione | 5.1±0.0 | 4.8±0.0 |
| Glutathione plus salt | 8.4±0.0 | 9.0±0.1 |
| Ascorbic acid | 5.1±0.0 | 4.9±0.1 |
| Ascorbic acid plus salt | 7.4±0.1 | 8.9±0.0 |
| Amino acid mixtures | 5.2±0.1 | 4.9±0.1 |
| Amino acids plus salt | 8.2±0.1 | 7.1±0.1 |
| F | 2266.13 | 2792.5 |
| P | ** | ** |
| LSD | 0.10 | 0.14 |
| Treatments | Phosphorus | |
|---|---|---|
| G124 | G119 | |
| Control | 0.009±0.0 | 0.013±0.0 |
| NaCl | 0.022±0.0 | 0.014±0.0 |
| Glutathione | 0.007±0.0 | 0.006±0.0 |
| Glutathione plus salt | 0.004±0.0 | 0.002±0.0 |
| Ascorbic acid | 0.008±0.0 | 0.004±0.0 |
| Ascorbic acid plus salt | 0.008±0.0 | 0.012±0.0 |
| Amino acid mixtures | 0.014±0.0 | 0.011±0.0 |
| Amino acids plus salt | 0.010±0.0 | 0.002±0.0 |
| F | 35099.2 | 24607.0 |
| P | ** | ** |
| LSD | 8.65 | 9.34 |
| Treatments | Potassium | |
|---|---|---|
| G124 | G119 | |
| Control | 12.6± 0.0 | 6.6± 0.1 |
| NaCl | 11.0± 0.1 | 5.6± 0.1 |
| Glutathione | 3.3± 0.3 | 6.9± 0.1 |
| Glutathione plus salt | 8.5± 0.1 | 6.0± 0.0 |
| Ascorbic acid | 5.3± 0.2 | 9.0± 0.0 |
| Ascorbic acid plus salt | 5.0± 0.2 | 8.4± 0.4 |
| Amino acid mixtures | 10.5±0.0 | 11.1± 0.0 |
| Amino acids plus salt | 5.8±0.1 | 8.5± 0.1 |
| F | 1516.8 | 10674.2 |
| P | ** | ** |
| LSD | 0.26 | 0.05 |
| Treatments | Sodium | |
|---|---|---|
| G124 | G119 | |
| Control | 1.53±0.03 | 0.39±0.07 |
| NaCl | 3.67± 0.06 | 1.56±0.07 |
| Glutathione | 0.67± 0.07 | 0.60±0.11 |
| Glutathione plus salt | 1.53±0.03 | 1.26±0.07 |
| Ascorbic acid | 0.37± 0.07 | 0.99±0.07 |
| Ascorbic acid plus salt | 0.67± 0.06 | 2.44±0.04 |
| Amino acid mixtures | 1.31± 0.09 | 1.02± 0.10 |
| Amino acids plus salt | 0.71± 0.09 | 0.94±0.04 |
| F | 1327.4 | 282.4 |
| P | ** | ** |
| LSD | 0.08 | 0.11 |
| Treatments | Nitrogen | |
|---|---|---|
| G124 | G119 | |
| Control | 0.88±0.007 | 0.65±0.003 |
| NaCl | 0.38±0.012 | 0.35±0.006 |
| Glutathione | 0.12±0.004 | 0.17±0.008 |
| Glutathione plus salt | 0.11±0.006 | 0.08±0.004 |
| Ascorbic acid | 0.67±0.009 | 0.91±0.001 |
| Ascorbic acid plus salt | 0.62±0.011 | 0.61±0.011 |
| Amino acid mixtures | 0.88±0.007 | 0.61±0.009 |
| Amino acids plus salt | 0.74±0.012 | 0.39±0.004 |
| F | 2802.8 | 4233.8 |
| P | ** | ** |
| LSD | 0.02 | 0.01 |
| Treatments | Potassium/sodium | |
|---|---|---|
| G124 | G119 | |
| Control | 8.2±0.1 | 16.7±0.1 |
| NaCl | 2.9±0.1 | 4.2±3.7 |
| Glutathione | 5.3±0.5 | 7.4±5.6 |
| Glutathione plus salt | 5.5±0.1 | 4.8±0.2 |
| Ascorbic acid | 14.7±3.0 | 9.2±0.7 |
| Ascorbic acid plus salt | 7.5±0.9 | 3.5±0.1 |
| Amino acid mixtures | 8.0±0.6 | 10.9±1.2 |
| Amino acids plus salt | 8.2±1.1 | 9.0±0.4 |
| F | 23.4 | 27.0 |
| P | ** | ** |
| LSD | 2.13 | 2.53 |

| Treatments | Protein nitrogen | |
|---|---|---|
| G124 | G119 | |
| Control | 2.5±0.01 | 2.1±0.01 |
| NaCl | 1.5±0.01 | 1.4±0.01 |
| Glutathione | 1.0±0.01 | 1.1±0.02 |
| Glutathione plus salt | 0.9±0.01 | 0.9±0.01 |
| Ascorbic acid | 2.1±0.01 | 2.6±0.00 |
| Ascorbic acid plus salt | 2.0±0.02 | 2.0±0.02 |
| Amino acid mixtures | 2.5±0.01 | 2.0±0.01 |
| Amino acids plus salt | 2.7±0.02 | 1.5±0.01 |
| F | 3315.4 | 4650.1 |
| P | ** | ** |
| LSD | 0.03 | 0.02 |

According to statistical analysis, salinity, glutathione, ascorbic acid, or an amino acid mixture with or without salt had a highly significant impact (P≤0.01) on the levels of phosphorus, potassium, sodium, the K/Na ratio, nitrogen, and protein nitrogen.
In the present study, salt stress significantly decreased all growth parameters, including fresh weight, dry weight, shoot height, root depth, succulence, and leaf area in both salt-tolerant and salt-sensitive barley cultivars (Irakoze et al., 2021). The reduction in leaf area under salt stress means that photosynthesis is decreased (Abdel-Farid et al., 2020; Munns & Tester, 2008). Such decrease may be partly due to decreased cell water potential associated with stomatal closure thereby, decreased CO 2 assimilation, besides NaCl toxicity and decreased availability of water. These results were in agreement with (Razzaque et al., 2009). The seedling stage revealed significant sensitivity to salinity stress, particularly in Giza 119, where root and shoot lengths experienced significant reductions (e.g., G119 root depth: 3.3±1.0 cm). The contrasting response in Giza 124 (e.g., G124 root depth: 5.7±0.8 cm) suggested cultivar-specific variations in tolerance. Notably, the application of glutathione, ascorbic acid, or amino acid mixtures showed their ameliorative effects, with Giza 124 exhibiting notable improvements in root (6.3±1,2, 8.0 ±1.0, 9.7 ±2.1 respectively) and shoot lengths (29.0±1.7, 26.0±1.7, 26.7±1.5) emphasizing the potential benefits of these additions in enhancing seedling growth under salt stress. The current findings supported those of (Asch et al., 2000) who studied various rice cultivars and found that the salt-tolerant cultivar (Giza 124) showed less dry matter degradation than the sensitive one. As anti-oxidant compounds, glutathione (GSH) and ascorbic acid (ASH) were found to be effective in raising all growth parameters, which may be related to their inhibitory effect on the uptake of Cl- and Na+ ions and their stimulatory effect on the uptake of the necessary elements, such as N, Mg, and Fe (Abd El-Hameid & Sadak, 2020; Munir et al., 2021). Rawia et al. (2011) reported that exogenous application of glutathione or ascorbic acid mitigated partially or completely the adverse effects of salt stress on the growth of canola seedlings and Marigold plants, respectively. In this study, the addition of a mixture containing a number of amino acids; arginine, ornithine and methionine has induced an increase in the shoot and root dry weights of salt stressed seedlings of each cultivar. In this regard (El-Shourbagy, 1964), showed a similar effect of amino acid mixture with the in vitro culture of tomato roots. However (Shams et al., 2014; El-Zohiri & Asfour, 2009), showed that the effect of polyamine precursors on the protein assimilation and cell formation of some potato cultivars can be attributed to the assimilation of gibberellins biosynthesis.
Salt stress induced a significant reduction in chlorophyll a (e.g., G124: 0.13±0.0, G119: 0.11±0.0) and chlorophyll b levels (e.g., G124: 0.09±0.0, G119: 0.12±0.0) in the two cultivars of barley which agreed with the results of (Taïbi et al., 2016) for two genotypes, high-yielding ‘Tema’ and low-yielding ‘Djadida’, commonly cultivated in Algeria. This decrease may be due to increased chlorophyllase activity as reported by (Nazarbeygi et al., 2011) and also may be caused by suppression of specific enzymes responsible for the synthesis of green pigments (Kaur et al., 2014; Mishra et al., 2006). In contrast, carotenoids were significantly increased in the salt stressed barley cultivars (e.g., G124: 0.20±0.0, G119: 0.21±0.0) and this may be due to their role in the protection of the photosystems by reacting with the lipid peroxidation products (Borghesi et al., 2011).
In Giza 124, glutathione and ascorbic acid with or without salt significantly increased chlorophyll a levels (e.g., 0.19±0.0 for glutathione, and 0.20±0.0 for glutathione with salt) under salt stress compared to the control (Chaturvedi et al., 2022). Amini and Ehsanpour (2005) found similar results with canola seeds. In comparison to the control, G119 showed an increase in carotenoids when treated with glutathione, ascorbic acid, or an amino acid combination with or without salt. Glutathione’s impacts on pigment levels may be due to their effects on either enhancing photosynthetic activities and chlorophyll biosynthesis or retarding chlorophyll degradation caused by oxidative stress. The addition of amino acids (polyamine precursors) has, on the other hand, resulted in an increase in chl b in both cultivars (Tyagi et al., 2023). Similar results have been reached with garlic plants (El-Shabasi et al., 2005), and sweet pepper (Tyagi et al., 2023) Al-Said & Kamal, 2008). This increase may be due to the role of amino acid mixture in detoxification of harmful accumulated of ROS in the thylakoid membrane during photosynthesis.
The current study showed a highly significant decrease in photosynthetic efficiency (Fv/Fm) under salt stress in both seedling and flowering stages, which can be attributed to photochemical quenching in PSII. Salt stress increased reactive oxygen species formation, which damaged PSII and organelles such as chloroplasts, mitochondria, and plasma membranes. As a result, PSII’s efficiency decreases. Several authors have reported comparable outcomes with several crop species under salt stress (Alnusairi et al., 2021). When glutathione, ascorbic acid, or an amino acid combination (polyamine precursors) were combined with salt, photosynthetic efficiency increased compared to the control. This could be related to their role in mitigating the harmful effects of salt via ROS scavenging.
There was a highly significant decrease in the total soluble carbohydrates in shoot and root of each cultivar under salinity stress compared with the control at the three studied growth stages. However, the salt-tolerant cultivar (Giza 124) has generally greater total soluble sugars in shoot and root respectively (30.9 ±0.1/21.8±0.0) compared to the salt-sensitive one, Giza 119 (22.7±0.2/9.6±0.1) and this agreed with the results obtained by Almodares et al. (2008) and Ashraf and Tufail (1995) in sunflower. In each barley cultivar, the decrease in the total soluble carbohydrates under salt stress may be due to that the building up in the chloroplast extent a direct toxic effect on photosynthetic processes (Munns & Tester, 2008) or to a reduction in CO2 assimilation rate and in the stomatal conductance. However, treatment with GSH or ASH has stimulated the accumulation of total soluble carbohydrates which are required as compatible solutes for osmoregulation in plants under water and salt-stresses (Hare et al., 1998). Accumulation of these compatible solutes reduces the osmotic potential in the cytoplasm and contributes to maintaining water homeostasis among several cellular compartments (Sairam & Tyagi, 2004), thus tuning the rate of photosynthesis to match the demand arising from grown inhibition.
The effects of glutathione or ascorbic acid on the accumulation of total soluble sugars can probably be attributed to their protective effects on the photosynthetic systems. However, GSH plays a protective role in salinity tolerance through the maintenance of the redox status (Chaparzadeh et al., 2004). Proline, an osmoprotectant, has been shown to accumulate more in plants in response to salinity (Ueda et al., 2007). Increased cellular proline levels are strongly correlated with the ability to withstand environmental challenges, such as salinity, and they can also act as an organic nitrogen reserve (Sairam & Tyagi, 2004). Proline levels rose in all barley cultivars when exposed to salinity (Somayeh et al., 2012), but it accumulated more in the salt-tolerant variety (Giza 124), which is related to its capacity to withstand salinity stress. The higher level of proline content in the two cultivars under salinity may be due to the expression of a gene encoding key enzymes of proline synthesis and low activity of the oxidizing enzymes which is controlled by either osmotic or salinity stress (Poury et al., 2023; Ma et al., 2015; Somayeh et al., 2012). Proline can act as an enzyme protector or a free radical scavenger. Results revealed that adding glutathione, ascorbic acid, or an amino acid combination reduced the level of proline. Such inverse correlation between these antioxidant compounds and proline in barley cultivars can be due to a deficit of common precursors which agreed with the results of Hoque et al. (2007) and Okuma et al. (2002) on Geum urbanum.
However, the tolerant cultivar was found to have larger levels of the ROS-scavenging enzymes catalase and peroxidase than the sensitive one, indicating that the antioxidant system is crucial for plant tolerance against environmental stresses (Challabathula et al., 2022). In this respect (Nawaz & Ashraf, 2007; Sairam et al., 2000), found that variations in the antioxidant systems of wheat and maize genotypes lead to differences in how the plants react to diverse stresses. According to the current findings, salt stress boosted the CAT and POD activities in both barley varieties. The salt-tolerant (Giza 124) showed a greater increase than the salt-sensitive one, showing that the former was a more effective scavenger for the H2O2 radical and provided superior protection against H2O2. Similar results have been observed with Beta maritime (halophyte) compared to the non-halophyte Beta vulgaris (Bor et al., 2003), and wheat (Sairam et al., 2002) differing in salt tolerance. However, the addition of glutathione, ascorbic acid, or an amino acid mixture to salt-treated barley cultivars significantly reduced the activities of catalase and peroxidase, indicating a compensatory or substituting effect for the already present antioxidant enzymes by directly scavenging H2O2 and other ROS.
Malonyldialdehyde (MDA), a criterion for evaluating salt injury, causes lipid peroxidation in a variety of plants (Ma et al., 2015; Somayeh et al., 2012). In response to salinity, both barley cultivars showed a highly significant increase in lipid peroxidation (at the seedling stage) and membrane leakage (at the seedling and Pre-flowering stages), with the salt-sensitive cultivar showing these effects more obvious than the salt-tolerant cultivar. The increase in MDA level when exposed to salt may be caused by oxidative damage to the chloroplasts and mitochondria or by antioxidants’ inability to completely neutralize and scavenge all of the active oxygen species produced when exposed to salt stress. The present results agreed with those of (Demiral & Türkan, 2005) for two rice cultivars (Chaparzadeh et al., 2004), for Calendula officinalis. Increasing lipid peroxidation during salt stress has been reported also (Xing et al., 2019) with Portulaca oleracea L.
In the two barley cultivars, MDA, as a product of the lipid peroxidation, has decreased with the addition of glutathione, ascorbic acid or amino acid mixture due to their compensating effects on the activities of antioxidant enzymes, acting as scavengers of cytotoxic H2O2 and reacting non-enzymatically with other ROS (Wang et al., 2014; Sairam et al., 2002). The amino acid mixture has reduced MDA content and membrane leakage which agreed with the results of (Zhang and Kirkham, 1996) for sorghum and sunflower seedlings, and Calndula plants (Baniasadi et al., 2018).
Several authors have reported increased levels of PAs when plants are exposed to diverse kinds of environmental stress (Jangra et al., 2023; Ramazan et al., 2022; Chattopadhayay et al., 2002; Bouchereau et al., 1999; Nayyar & Chander, 2004). The present results indicated that under salt stress, PUT increased in the tolerant cultivar (Giza 124) whereas Spd and Spm increased in the sensitive cultivar. In contrast to these findings (Krishnamurthy & Bhagwat, 1989), found that during salt stress, rice cultivars that were salt-resistant acquired more Spd and Spm than sensitive ones. Similar results were reported by De Oliveira et al. (2020). Additionally, they noted that sensitive rice accumulated more Put under salt than tolerant rice (De Oliveira et al., 2020; Erdei et al., 1996) indicate that Spd and Spm accumulation in sorghum is one of the adaptive responses to salt stress. Chattopadhayay et al. (2002) and Willadino et al. (1996) proposed that accumulation of Spd and Spm may contribute to stress tolerance, while PUT accumulation may have no positive effect under salinity stress. Besides, it has been reported that, mainly in cereals, Put accumulation seemed to be related especially to the activation of arginine decarboxylase under stress conditions (Bouchereau et al., 1999).
Salt stress has increased phosphorus concentration in the shoot and root of each barley cultivar which agreed with the results of (Taban, 2000; Yahya, 1998). According to Roberts et al. (1984) the increase of P level in maize is the result of enhanced rates of uptake by the roots and of translocation to the shoots and not a concentration effect due to growth depression or may be due to ion imbalance between the uptake of sodium and ions including phosphorus (Sacala et al., 2016; Lutts et al., 1999). However, under the effect of glutathione, ascorbic acid, or amino acid mixture with or without salt, phosphorus content decreased in each cultivar. This decrease may be due to the ameliorating effect or controlling the uptake of phosphorus which is linked with pH.
Results showed that Giza 124 showed a greater accumulation of sodium under salinity compared to Giza 119, and this agreed with the results of (Flowers, 2004; He & Cramer, 1992). They found that maize shoots of Giza 2 (salt-tolerant) have greater levels of Na+ compared to those of Tri-hybrid 321(salt sensitive) under NaCl treatments. Furthermore, the greatest accumulation of sodium by plants at high salt concentrations may be attributed to the fact that selective salt absorption may be replaced by passive absorption which causes abnormal accumulation of salts in plant organs (Bouchereau et al., 1999). They suggested that under salinity sodium influx across the plasmalemma to the vacuole might play a major role in permitting turgor maintenance (Yang et al., 2019).
It may be suggested that the cultivar Giza 124 had the ability to sequester Na+ into the vacuole more efficiently than Giza 119. Several reports indicated that the absence of ion compartmentation may contribute to the toxic effects of ions in the shoot of sensitive plants (Flowers & Yeo, 1997). However, K+ concentration and consequently K+/Na+ ratio were decreased under salt-stress in the leaves of each cultivar (Yu et al., 2023). These decreases could be due to the effect of Na+ on K+ transport into the xylem or an inhibition of uptake processes (Gu et al., 2016; Yan et al., 2013). Again, decreasing in potassium content can be due to an antagonistic effect between sodium and potassium which has been confirmed by de Azevedo Neto and Tabosa (2000). These antagonistic relations between Na+ and K+ may be taken as an indication of the role played by hormones in modifying K+/Na+ selectively under salt stress (Alpaslan & Gunes, 2001). Studies with the two barley cultivars showed that salinity has a significant reducing effect on the K+ concentration in both shoot and root, which can be referred to the competition and resultant selective uptake between K+ and Na+ which causes an increase in uptake of Na+ at the cost of K+ or due to a decrease in sink size with the higher concentrations of NaCl, which strongly inhibited shoot and root growth (Ashrafuzzaman et al., 2000). Gebauer et al. (2003) reported that K+ accumulation was reduced in the stem and increased in leaves and roots with increasing concentration of NaCl ((Falhof et al., 2016; Flowers & Yeo, 1997). Epstein (1998) claimed that enhanced K+ uptake can be an adaptive mechanism that allows the cell to evade K+ starvation in the presence of high NaCl concentrations, which does not agree with the present study. Our findings align with the conclusion reported by Behzadi Rad et al. (2021).
Results showed an increase in shoot-nitrogen content in each barley cultivar. Flanagan and Jefferies (1988) showed that when plants are subjected to high salinity, higher nitrogen content was associated with osmotic solute or an accumulation of nitrate ions or increased protein degradation (Zhang et al., 2023). Application of glutathione, ascorbic acid or amino acid mixture with or without salt has led to a reduction in nitrogen content in the shoot of Giza 124 compared to the control and the treatment of the salt alone, while in Giza 119, glutathione without salt and ascorbic acid with salt caused a higher nitrogen content compared to control and salt alone. This difference can be due to impairment of nitrogen metabolism in the sensitive cultivars while the tolerant one was able to use nitrogen for enzymatic defense system against salt. In contrast, root-nitrogen content decreased with salinity in each cultivar.
Concerning the yield, it is noticeable that Giza 124 exhibited a favorable response to treatments including ascorbic acid (1636.7±32.1 spikes per plant) and amino acid (1561.7±33.3 spikes per plant), suggesting that these additives may improve reproductive performance under conditions when salinity-induced stress is present. The present results showed that, in both cultivars, NaCl stress resulted in a reduction in grain weight in both cultivars which can be confirmed with the results of Sairam et al. (2000) and Sohrabi et al. (2008) for chickpea and (Taffouo et al., 2009) for some cowpea (Vigna unguiculata), and (Saleethong et al., 2013) for rice. Grain weight reduction can be related to the disturbance in the translocation due to toxic ions or a reduction in the photosynthesis, imbalance in mineral uptake, protein synthesis or carbohydrate metabolism (Farooq et al., 2017; Al-Garni, 2006). However (Zeng et al., 2002, p. 2), reported that the few differences in grain weight of rice (Oryza sativa L.) genotypes under salinity stress can be related to plant genus and genotype.
The negative impact of salinity stress on barley grain production was evident at the vital yield stage, as both cultivars showed considerable declines in the weight of grains per plant (G124: 860.026.5 mg/plant) and (G119:1141.7±7.63 mg/plant. However, ascorbic acid, glutathione, or amino acid combinations applied strategically showed encouraging results, reducing the adverse effect on yield (Alami-Milani & Aghaei-Gharachorlou, 2015; El-Beltagi et al., 2020). This demonstrates how certain compounds can increase barley yield even when salinity stress is occurring. Analyzing physiological responses, the decrease in total soluble carbohydrates was evident under salinity stress, although glutathione application showed a significant increase (e.g., G124: 23.6±0.0 mg/g dry wt.). Proline content, a key indicator of stress response, increased significantly under salinity (Rahneshan et al., 2018) and was further elevated with the addition of glutathione, ascorbic acid, or amino acid mixtures, emphasizing their role in enhancing stress tolerance mechanisms (Parvaneh et al., 2012). Mineral analysis showed compex responses in phosphorus, potassium, sodium, nitrogen, and protein nitrogen content. The intricate balance of these elements, as indicated by the potassium/sodium ratio (e.g., G124: 5.5±0.1), played a crucial role in determining the plant’s ability to withstand salinity stress. The significant impact on protein nitrogen content (e.g., G119: 1.5±0.01) highlighted the need for a nuanced understanding of nutrient dynamics under stress conditions.
The study showed the negative impact of salt stress on plant growth, emphasizing the efficacy of antioxidants like glutathione, ascorbic acid, and amino acids in mitigating these effects. These antioxidants enhanced nutrient uptake while inhibiting harmful ion absorption, contributing to improved growth parameters. Plant pigments changed under salt stress, with decreased chlorophyll levels and increased carotenoids, suggesting a defense mechanism against lipid peroxidation. Giza 124, a salt-tolerant cultivar, exhibited higher total soluble carbohydrate levels and improved photosynthetic efficiency compared to Giza 119. In addition, the study highlighted the importance of antioxidants, proline regulation, and ROS-scavenging enzymes in enhancing salt tolerance. It also showed the potential of glutathione in alleviating salinity’s impact on barley growth. The study’s insights contribute to understanding barley responses to salt stress, with future perspectives emphasizing long-term evaluations and practical applicability of the suggested treatments and the salt-tolerant cultivar (Giza 124). Practical insights could be obtained through field trials conducted in a variety of settings and integrated into precision agriculture procedures. Research in molecular and genetics may reveal underlying systems that aid in the creation of genetically engineered crops.
Figshare: Spreadsheets, https://doi.org/10.6084/m9.figshare.22082354.v1 (El Dakkak, 2023).
This project contains the following underlying data:
- Catalse (seedling).csv
- Chlorophyll (preflowering G119).csv
- Chlorophyll (preflowering).csv
- Chlorophyll-Seedling Stage G119.csv
- Chlorophyll-Seedling Stage-G124.csv
- Growth Criteria Seedling Stage-G124.csv
- Growth Criteria Seedling Stage-G119.csv
- Lipid Peroxidation.csv
- Membrane Leakage.csv
- Nitrogen (yield).csv
- Nitrogen (seedling).csv
- Peroxidase (seedling).csv
- Phosphorous (seedling).csv
- Phosphorous (yield).csv
- Polyamines.csv
- Potassium.csv
- Proline Content (seedling stage).csv
- Proline (yield).csv
- Protein Nitrogen.csv
- Sodium-Potassium.csv
- Succulence – Root.csv
- Succulence – Shoot.csv
- Total Soluble Carbohydrates (Preflowering).csv
- Total Soluble Carbohydrates (seedling).csv
- Total Soluble Carbohydrates (yield).csv
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
I would like to express my deep gratitude to my late advisor Prof. Dr. Mohamed Nabih El-Shourbagy, Professor of Plant Physiology, Botany Department, Faculty of Science, Tanta University for his kind supervision, great help, valuable suggestions and advice during the progress of this work. I am greatly indebted to Prof. Dr. Fatma A. El-Shintinawy, Professor of Plant Physiology, Botany Department, Faculty of Science, Tanta University for her kind assistance during the initial stage of this thesis. I would also want to thank Late Prof. Dr. Wedad Abd El-Aziz Kasim, Professor of Plant Physiology, Botany Department, Faculty of Science, Tanta University for her help and encouragement as well as for the critical reading of manuscripts.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Abdelrhim A, Hemeda N, Mwaheb M, Omar M, et al.: The role of Trichoderma koningii and Trichoderma harzianum in mitigating the combined stresses motivated by Sclerotiniasclerotiorum and salinity in common bean (Phaseolusvulgaris). Plant Stress. 2024; 11. Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Stress Plant Physiology
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Stress Physiology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
I cannot comment. A qualified statistician is required.
Are all the source data underlying the results available to ensure full reproducibility?
No source data required
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Kumar D, Dhankher OP, Tripathi RD, Seth CS: Titanium dioxide nanoparticles potentially regulate the mechanism(s) for photosynthetic attributes, genotoxicity, antioxidants defense machinery, and phytochelatins synthesis in relation to hexavalent chromium toxicity in Helianthus annuus L.J Hazard Mater. 2023; 454: 131418 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Stress Physiology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 18 Jan 24 |
read | read | |
|
Version 1 10 Mar 23 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)