Keywords
Brain tumours, gliblastoma, recurrence, heterogeneity, tumour microenviroment, IDH, temozolomide
This article is included in the Oncology gateway.
This article is included in the Cell & Molecular Biology gateway.
This article is included in the Genomics and Genetics gateway.
This article is included in the Glioblastoma Heterogeneity collection.
Brain tumours, gliblastoma, recurrence, heterogeneity, tumour microenviroment, IDH, temozolomide
Glioblastoma (GBM) is the most common and aggressive form of primary brain cancer (Ostrom et al., 2019; Wen et al., 2020). Current treatment approaches combine surgical resection, followed by radiotherapy and chemotherapy (Stupp et al., 2005; Stupp et al., 2009). Tragically, tumour recurrence is inevitable. GBM tumours are highly infiltrative, and complete surgical resection is not possible. Subpopulations of tumour cells escape resection, develop resistance to current treatments, and ultimately divide and repopulate the brain. There is no standard of care once tumour recurrence has occurred. Prognosis is poor, with a median survival rate of 15 months post-initial diagnosis, which has not improved in the past 15 years (van Linde et al., 2017; Ostrom et al., 2019; Wen et al., 2020). New therapies that effectively target renegade GBM cells are desperately needed. However, this requires greater insight into the genetic, transcriptional and microenvironmental changes that occur under therapeutic conditions that allow GBM cells to evade treatment and foster the persistence and expansion of therapy-resistant tumour cell populations.
GBM tumours exhibit a high level of heterogeneity both between patients and within an individual’s tumour. Somatic alterations contribute to this heterogeneity, as clonal populations inheriting mutations that favour tumour progression arise over time (Brennan et al., 2013; Sottoriva et al., 2013; Wang et al., 2016; Muscat et al., 2017). This is proposed to promote the collective resistance of the tumour to therapy as selection pressures enrich for treatment-resistant tumour cell clones. Additionally, tumour heterogeneity impedes the development of effective treatment options, as not all patients respond favourably to the same treatment. Historically, mutations observed in the isocitrate dehydrogenase (IDH1 and IDH2) genes denoted two molecularly and clinically distinct forms of GBM, IDH wildtype (IDHwt; primary GBM) and IDH mutant (IDHmut; secondary GBM) (Parsons et al., 2008; Han et al., 2020). IDHwt tumours accounted for 95% of GBM cases and correlated with poorer prognoses. In 2021, the updated WHO Classification of Tumours of the Central Nervous System restricted the diagnosis of GBM to IDHwt tumours, with IDH-mutant tumours now classified as astrocytomas or oligodendrogliomas depending on the respective absence or presence of 1p19q co-deletions (Louis et al., 2021). As such, IDHwt tumours will be the focus of this commentary. Other common genetic alterations in GBM include amplifications of EGFR and PDGFR, activating mutations in the TERT promoter, loss of PTEN and NF1, the simultaneous gain of chromosome 7 and loss of heterozygosity of chromosome 10, and aberrations in RTK/Ras/PI3K signalling pathways, most of which negatively correlate with patient survival (Cancer Genome Atlas Research Network, 2008; Brennan et al., 2013; Barthel et al., 2019). Longitudinal mutational analysis has identified changes in molecular alterations from primary to recurrent GBM, although the reports have been conflicting (Kim et al., 2015; Wang et al., 2016; Muscat et al., 2017; Barthel et al., 2019; Draaisma et al., 2020). For example, several studies have identified reduced expression or loss of EGFR mutations in recurrent GBM, suggesting clonal replacement that favours reduced EGFR (Wang et al., 2016; van den Bent et al., 2015; Cioca et al., 2016). However, the opposite has also been observed (Neilsen et al., 2019). Conversely, the Glioma Longitudinal Analysis (GLASS) Consortium reported little evidence of recurrence-specific gene alterations, with the clonal representation of driver mutations remaining similar in primary and matched recurrence tumours (Barthel et al., 2019). Thus, rather than identifying a stereotyped trajectory in genetic evolution during therapy, they observed that most glioma tumours stochastically evolved along patient-specific paths (Barthel et al., 2019). Multiple studies have, however, identified a treatment-associated hypermutant genotype induced by the administration of alkylating agents, such as temozolomide which is part of the standard of care for GBM patients (Johnson et al., 2014; Wang et al., 2016; Barthel et al., 2019; Choi et al., 2018). Contrary to previous publications (Johnson et al., 2014), Barthel et al. (2019) did not identify a detrimental effect of the hypermutator phenotype on patient survival, so the impact of this genotype remains unclear. While genetic perturbations underlie GBM pathogenesis, it is clear that additional factors beyond somatic alterations influence therapy resistance and GBM recurrence.
In recent years, bulk RNA sequencing studies of gliomas have defined three reproducible transcriptional TCGA subtypes: classical, mesenchymal, and proneural (Verhaak et al., 2010, Wang et al., 2017). These subtypes partially correlate with genetic alterations; for example, alterations in EGFR are enriched in classical subtype tumours, while PDGFRA alterations are more common in proneural tumours (Verhaak et al., 2010). Subtype-associated differences in disease outcomes have also been observed, with mesenchymal tumours generally exhibiting a poorer prognosis, highlighting this molecular characterisation as a potentially useful clinical tool (Colman et al., 2010; Wang et al., 2017). Longitudinal analyses have demonstrated that subtype switching can occur over the time of tumour progression (Phillips et al., 2006; Halliday et al., 2014; Wang et al., 2016; Wang et al., 2017). Proneural to mesenchymal subtype switching upon disease recurrence is associated with increased resistance to therapy (Bhat et al., 2013, Ozawa et al., 2014, Phillips et al., 2006). However, the evolutionary temporal dynamics of the subtypes and the resultant recurrence-specific features are less known. Importantly, multiple subtypes can co-exist within the same tumour, as shown by multi-region bulk and single-cell RNA sequencing (Sottoriva et al., 2013, Patel et al., 2014), which contributes to intratumoral heterogeneity. Single-cell transcriptional analysis has allowed the interrogation of malignant cell subpopulations that give rise to GBM with diverse cellular compositions. Neftel et al. (2019) identified that GBM neoplastic cells exist in a limited set of transcriptional cellular states, including more stem cell-like states (neural-progenitor-like and oligodendrocyte-progenitor-like) and more differentiated states (astrocyte-like and mesenchymal-like). These states are plastic, and specific genetic alterations influence the relative frequency of each state in a tumour. For example, EGFR modifications are associated with a higher frequency of astrocyte-like cells. Beyond somatic alterations, the neoplastic transcriptional state also appears to be influenced by additional factors, such as the local microenvironment. For example, the Mesenchymal neoplastic cell state is associated with high expressions of hypoxia genes and the TCGA-MES subtype is enriched in macrophages and microglia (Neftel et al., 2019). Thus, multiple factors likely influence the dynamic evolution underpinning glioma recurrence.
Resistance to therapy is also influenced by interactions of tumour cells with the microenvironment of the brain (Perrin et al., 2019). The brain tumour microenvironment includes stromal cells of the native brain parenchyma, such as neurons, astrocytes, oligodendrocytes, pericytes and microvascular endothelial cells, as well as infiltrating and resident immune cells, such as myeloid cells (e.g., macrophages and microglia). Interactions between tumour cells and stromal cells in the microenvironment can elicit both pro- and anti-tumorigenic effects. For example, subpopulations of microglia can mediate either pro-inflammatory functions, facilitating the infiltration of cytotoxic T cells and decreased tumour growth, or anti-inflammatory functions, creating an immunosuppressive tumour environment that promotes tumour progression (Geribaldi-Doldán et al., 2021). GBM tumour cells can modify these interactions to fit specific survival needs allowing adaptation under environmental and therapeutic-induced stress. This selection pressure facilitates the formation of distinct regions across the tumour landscape, containing different populations of both malignant and stromal cells, distinguishable by anatomical features from histology analysis (Puchalski et al., 2018; Yu et al., 2020; Zadeh Shirazi et al., 2021; Ravi et al., 2022). Recent studies have highlighted that tumour recurrence is associated with transitions in the stromal landscape of tumours, including the immune microenvironment, that can favour tumour progression (Wang et al., 2017; Gangoso et al., 2021). Thus, understanding how GBM tumours hijack their microenvironments to promote progression will allow the identification of novel therapeutic targets beyond the driver mutation profile of tumours.
Most studies using clinical GBM specimens have focused on primary tumour tissues captured at first surgical resection. Second and third surgical resections in GBM patients are infrequent; thus, limited tissue resources are available to study recurrent tumour interactions (Ringel et al., 2016; GLASS Consortium 2018). GBM tumours display high levels of inter-patient heterogeneity, which emphasises the need for large data sets to identify unifying trends in disease evolution. The Glioma Longitudinal AnalySiS (GLASS) consortium was initiated in 2015 to bring together leading experts across dispensary fields to accelerate understanding of glioma tumour evolution and expose therapeutic vulnerabilities (GLASS Consortium 2018). To achieve this, GLASS has curated an extensive, publicly accessible molecular and clinical longitudinal data resource from patients with multi-sampled glioma tumours. The GLASS consortium previously employed a wide-scope approach and performed genomic profiling of matched primary and recurrent tumours from 222 patients to track clonal dynamics (Barthel et al., 2019). For Varn et al. (2022), this data was expanded by integrating longitudinal genomic data with matched transcriptomic data, comprising a total of 128 IDHwt glioma patients with RNA sequencing performed for two or more time points. Moving towards a more complex, systems biological appraisal of glioma evolution, the authors provide a more comprehensive insight into the dynamic transcriptional and cellular composition changes glioma tumours undergo in response to current therapy.
Previous studies have identified an association between changes in the tumour tissue microenvironment and tumour cell transcriptional state (Neftel et al., 2019). As such, the authors sought to identify longitudinal trends in transcriptional programs in recurrent tumours and their association with the physical structure and cell composition of the tumour microenvironment.
The authors observed that TCGA transcriptional subtype switching occurred in almost half of patients (49%) (Varn et al., 2022). Classical to mesenchymal was the most common transition, contributing to a slight decrease in classical tumours in favour of an overall increase in mesenchymal tumours across the cohort. To interrogate specific changes in the microenvironment and the tumour cell transcriptional state that may underlie this transition, the authors deconvoluted the GLASS gene expression dataset using CIBERSORTx (Newman et al., 2019). They identified 12 cell states representing both neoplastic and non-malignant cell compartments of the brain microenvironment. As observed in previous single-cell studies (Neftel et al., 2019), the neoplastic population could be further subdivided into distinct transcriptional states, including a differentiated-like state and two stem-like states (segregated by cell cycle activity). The relative abundance of the 12 cell states also varied across the TCGA transcriptional subtypes, supporting findings in previous publications (Neftel et al., 2019; Wang et al., 2017). By comparing matched primary and recurrent tumour transcriptomes, Varn et al. (2022) also observed an increase in oligodendrocyte populations at recurrence, independent of the extent of surgical resection. The role of oligodendrocytes within the tumour microenvironment has yet to be extensively studied. However, recent single cell (sc) RNA sequencing and in vitro studies have suggested bi-directional communication between tumour cells, non-malignant oligodendrocytes and oligodendrocyte precursor cells in the microenvironment, which may contribute to tumour progression (Hide et al., 2018; Caruso et al., 2020). The authors also observed a significant decrease in differentiated-like neoplastic cells at recurrence and little difference in the abundances of stem-like and proliferating stem-like cell populations (Varn et al., 2022), supporting the concept that relative cell compositions of the microenvironment shift as a GBM tumour evolves.
Using Ivy GBM atlas project (Ivy GAP) data (Puchalski et al., 2018), which comprises bulk RNA sequencing from five micro-dissected histological features along with multiplex immunofluorescence, the authors identified that cell state composition was more closely associated with histological features than with the patient from which they were derived (Varn et al., 2022). For example, ‘leading-edge’ features were enriched in oligodendrocytes and stem-like neoplastic cells, while differentiated-like neoplastic cells were enriched in ‘pseudopalisading cells around necrosis’ and ‘cellular tumour’ features (Varn et al., 2022). This suggests that changes in histological features may influence cell composition at tumour recurrence. After using Ivy GAP histological feature gene signatures to deconvolute the GLASS dataset, a correlation between longitudinally changing neoplastic cell state abundances and histological features was detected (Varn et al., 2022). A recent spatial transcriptomics study by Ravi et al. (2022) has since supported an association between cell state composition and physical tumour structure. The neoplastic cell states identified by Neftel et al. (2019) were also spatially segregated across distinct tumour tissue regions and hypothesised as an indication of reactive adaptation to environmental stress. While Varn et al. (2022) concluded that in most cases, the subtype switch observed at recurrence was attributable to changes in histological feature composition over time, they noted that some changes appear to be independent of this phenomenon. For example, tumours undergoing proneural-to-mesenchymal transition appeared to lose stem-like cells independently of the histological feature composition, indicating that other factors may influence cell state abundances at recurrence (Varn et al., 2022). Moreover, regardless of transcriptional subtype transition, IDHwt tumours showed significantly higher ‘leading-edge’ content at recurrence (Varn et al., 2022), which suggests that this may be a general feature of recurrent tumours.
While the TCGA molecular subtypes have been a critical asset in interrogating the clinically significant molecular differences across GBM patients at diagnosis, their application to recurrent tumours may be more limited. Stratifying recurrent GBM tumours into reoccurring molecular subtypes may help determine patient-specific treatment plans for secondary tumours and identify trajectories of therapy-driven evolution as cells adapt to resist treatment. Three distinctive recurrence-associated phenotypes, i.e., neuronal, mesenchymal and proliferative, are driven by somatic mutations, interactions with microenvironment cells and changes to histological feature composition (Varn et al., 2022). Additionally, some tumours exhibited multiple phenotypes highlighting that recurrent tumours are still highly heterogeneous.
The neuronal recurrence-associated phenotype was the most common subtype observed in 66% of tumours in the GLASS IDHwt cohort (Varn et al., 2022). This phenotype was not associated with any significant impacts on patient survival. The neuronal recurrence phenotype was characterised by increased neuronal signalling gene signatures in stem-like neoplastic cell populations and an increase in leading-edge histological features. Varn et al., hypothesised that these characteristics result from enhanced tumour-neuron interactions as recurrent tumours invade the native brain parenchyma (Varn et al., 2022). To support this, they utilised scRNA-seq from spatially defined GBM regions previously produced by Yu et al. (2020) to confirm that neoplastic cells collected from the invasive rim had significantly higher expression of the stem-like neoplastic cell recurrence signature versus those collected from the tumour core. To further validate this observation, the authors performed multiplex immunofluorescence in recurrent glioma using the neuronal marker SNAP25 (Varn et al., 2022). They identified a high number of neurons and SNAP25 + neoplastic cells (SOX2+) in the infiltrating tumour region compared to the cellular tumour region, lending support to increased signalling between neoplastic cells and neighbouring neural cells at recurrence. Studies exploring neuron-to-glioma synapses have recently attracted significant research interest due to the potential influences on tumour proliferation and invasion (Venkataramani et al., 2019; Venkatesh et al., 2019). More recently, Venkataramani et al. (2022) employed patient derived xenograft (PDX) models to show that the leading edge of GBM is enriched in neoplastic cells expressing neuronal and neural-progenitor-like cell states, supporting Varn et al. (2022) observations. They also observed that leading-edge GBM cells utilise neuronal migration pathways to invade the native parenchyma where they receive synaptic input from neurons to enhance migration (Venkataramani et al., 2022). Thus, the neuronal recurrence subtype may be particularly vulnerable to therapeutic interventions that disrupt synaptic communication between tumour cells and neurons.
The mesenchymal recurrence phenotype was observed in 45% of IDHwt tumours in the GLASS cohort and was significantly associated with poorer patient outcomes than the non-mesenchymal recurrence phenotypes (Varn et al., 2022). This supports previous observations using TCGA data where mesenchymal subtype tumours were associated with treatment resistance and reduced survival (Carro et al., 2010; Bhat et al., 2013; Wang et al., 2017). The mesenchymal recurrence phenotype was characterised by an abundance of differentiated-like neoplastic cells expressing a mesenchymal-like signature and showed an enrichment of myeloid cells (Varn et al., 2022). The authors deconvoluted TCGA data to compare myeloid-specific gene expression profiles between mesenchymal and non-mesenchymal transcriptional subtypes to determine whether neoplastic and tumour-infiltrating myeloid cell interactions may be responsible for driving the transition of recurrent tumours towards this subtype (Varn et al., 2022). Intriguingly, mesenchymal tumours displayed a distinct myeloid-specific gene expression profile, exhibited an immunosuppressive phenotype and were enriched in chemokine signalling and lymphocyte chemotaxis functions. Following transcriptional subtype transitions over time, a significant induction of this signature was identified in recurrent tumours undergoing a mesenchymal transition (Varn et al., 2022). Using the IVY gap data (Puchalski et al., 2018), Varn et al., identified that this specific mesenchymal-myeloid signature was most associated with the ‘pseudopalisading cells around necrosis and microvascular proliferation’ histological features, which are features that are enriched with blood-derived macrophages. Thus, the authors hypothesised that some blood-derived macrophages directly interact with mesenchymal neoplastic cells at these specific regions (Varn et al., 2022). To test this, Varn et al., screened for candidate ligand-receptor pairs expressed in mesenchymal neoplastic cells and myeloid cells that specifically associate with mesenchymal transitions over time. This identified oncostatin M (OSM), expressed by myeloid cells, and oncostatin M receptor (OSMR), expressed by differentiated-like neoplastic cells, as the top candidate genes. Using multiplex immunofluorescence, the co-localisation of OSM+ myeloid cells and OSMR+ neoplastic cells around blood vessels was confirmed in mesenchymal IDHwt glioma samples (Varn et al., 2022). No co-localisation patterns were observed in the classical subtype tumours. Association between microglia/macrophage OSM expression and induction of mesenchymal-like expression programs in glioma has been reported by previous studies and proposed to be induced via the upregulation of STAT3 signalling in neoplastic cells (Natesh et al., 2015; Junk et al., 2017; Hara et al., 2021; Chen et al., 2021). OSM is a cytokine belonging to the IL-6 family, which is secreted by macrophages and microglia in the brain to regulate inflammatory responses (Modur et al., 1997). There is contradictory evidence for the role of OSM in glioma progression with some studies suggesting it exhibits anti-tumorigenic effects (Friedrich et al., 2001), while others have reported pro-invasive properties and an overall negative association between OSM expression and glioma patient survival (Natesh et al., 2015, Chen et al., 2021). Beyond the influence of stromal cells in the microenvironment, Varn et al. (2022) also observed that specific somatic alterations, such as NF1, EGFR or PDGFRA, are associated with mesenchymal transitions. These somatic alterations also affected non-neoplastic cell abundance; for example, NF1 mutants exhibited increases in granulocytes and myeloid cells, supporting previous observations (Wang et al., 2017). Thus Varn et al. (2022) have identified a complex interplay of microenvironmental and genetic factors that drive tumour transition towards the more aggressive mesenchymal subtype. Exploring potential vulnerabilities in tumour-myeloid interactions may be one avenue that can be exploited to improve patient survival.
The proliferative recurrence phenotype, characterised by increased proliferating stem-like neoplastic cells, was identified in 37% of IDHwt tumours (Varn et al., 2022). By analysing orthogonal DNA and RNA sequencing data of primary and matched recurrent tumour pairs, Varn et al. (2022) identified an increase in proliferating stem-like neoplastic cells associated with a hypermutation genotype at recurrence. As previously established, alkylating agents, such as Temozolomide, can induce hypermutation (Wang et al., 2016; Johnson et al., 2014). Using multiplex immunofluorescence, Varn et al. (2022) confirmed an increase in proliferating stem-like cells (SOX2+/Ki67+ cells) in recurrent IDHwt tumours relative to matched initial tumour tissue. Although the authors identified a positive association between proliferating stem-like cells and microvascular proliferation across the GLASS cohort, no corresponding increase in this histological feature was observed in hypermutant recurrence tumours (Varn et al., 2022). This suggests that factors other than the physical structure of the tumour, such as genetic alterations, influence the relative abundance of proliferating stem-like cells. Hypermutant recurrent tumours have been associated with an increased frequency of CD8+ T cells when compared to their primary tumours, contributing to a potentially more immunologically reactive microenvironment (Wang et al., 2017). However, Varn et al. (2022) did not observe any significant changes in the relative abundances of stromal cells, including T-cells, between hypermutated recurrences and their matched initial tumours. Treatment-associated hypermutation is more frequently observed among IDHmut tumours than IDHwt tumours suggesting sensitivity to mutation upon loss of IDH function (Barthel et al., 2019). Indeed, the authors detected the proliferative recurrence phenotype in 53% IDHmut tumours, which is negatively associated with patient survival (Varn et al., 2022). No such survival association was seen in IDHwt tumours. The clinical relevance of the hypermutation phenotype is still elusive for IDHwt tumours and highlights the need for future studies into the impact of hypermutation on disease progression.
One major obstacle to effective treatment for IDHwt GBM tumours is the high cellular heterogeneity that is a feature of both primary and recurrent tumours. Molecular characterisation of primary tumours, such as the gene expression-based TCGA subtypes, has dramatically advanced our understanding of clinically relevant molecular differences across patient groups associated with therapy responses. However, the translation of these findings to improve patient outcomes is still limited. In this study, the GLASS consortium has more comprehensibly mapped the molecular characteristics of tumours over patients’ disease trajectories (Varn et al., 2022). They identified that genetic and environmental factors shape the composition of cell states in IDHwt tumours. This has provided a framework for recurrent GBM molecular stratification, identifying three recurrence-specific phenotypes which may display distinct therapeutic vulnerabilities (Figure 1).
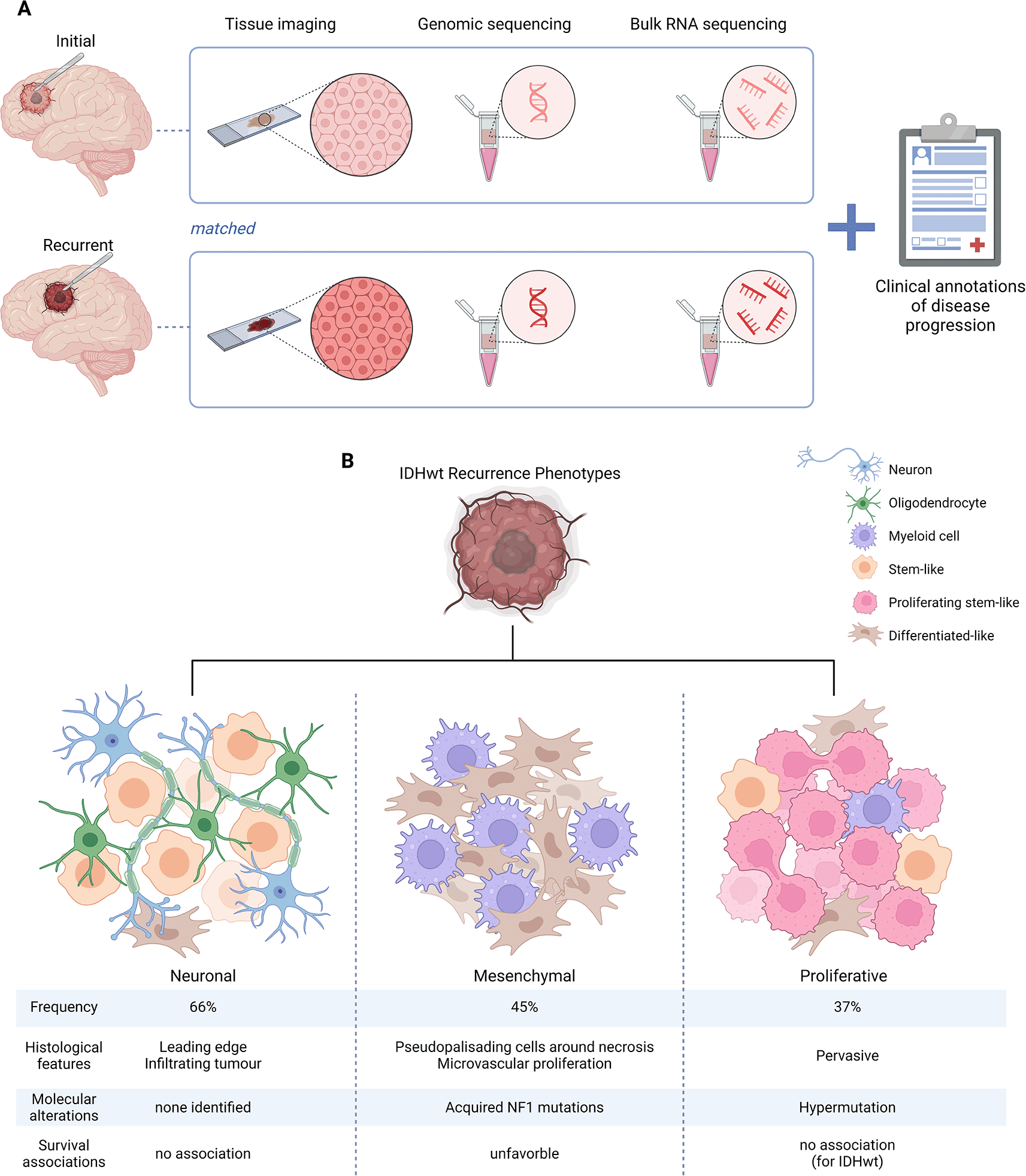
A) The Glioma Longitudinal AnalySiS (GLASS) consortium cohort comprises a comprehensive molecular data resource of glioma tumour tissues, including clinical annotations, imaging and genomic data sampled at multiple time points along the course of glioma progression. For Varn et al. (2022), this data was expanded by integrating matched longitudinal bulk transcriptomic data. B) Recurrent IDHwt gliomas can be stratified into three recurrence-specific phenotypes: neuronal, mesenchymal, and proliferative. Each phenotype is associated with specific histological features, molecular alterations, and clinical outcomes. Phenotype presentation across the GLASS cohort varies in frequency, and notably, some recurrent tumours can exhibit multiple phenotypes at once, indicating intra-tumour heterogeneity. Figure created with BioRender.com.
Future studies should further elucidate how the interplay between tumour cell states, driver mutations and the microenvironment can be exploited to skew tumours towards a sensitised molecular subtype that can be effectively treated with radiotherapy and/or chemotherapy. Achieving this aim will require experimental GBM models that better recapitulate the parental tumour, assess therapeutics along the clinical time course, and build predictive treatment models (Schäfer et al., 2019; Gomez et al., 2019; Lenin et al., 2021). Incorporating such methods into standard-of-care practice will help to facilitate personalised therapies for GBM patients that lead to better patient outcomes.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the topic of the opinion article discussed accurately in the context of the current literature?
Yes
Are all factual statements correct and adequately supported by citations?
Yes
Are arguments sufficiently supported by evidence from the published literature?
Yes
Are the conclusions drawn balanced and justified on the basis of the presented arguments?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: glioblastoma, tumor plasticity, immunotherapy, tumor microenvironment, pre-clinical models of cancer
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |
|---|---|
| 1 | |
|
Version 1 13 Jan 23 |
read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)