Keywords
Mast cell, Aplastic anaemia, MDS-h, Hypocellular marrow, Bone marrow niche, CD34, Hematopoietic stem cell.
This article is included in the Manipal Academy of Higher Education gateway.
This article is included in the Oncology gateway.
Mast cell, Aplastic anaemia, MDS-h, Hypocellular marrow, Bone marrow niche, CD34, Hematopoietic stem cell.
Haematological disorders characterised by hypocellular marrows like aplastic anaemia (AA) and hypocellular myelodysplastic neoplasm (MDS-h) are diagnostic challenges for haematologists as AA, MDS-h, other immune cytopenias, paroxysmal nocturnal haemoglobinuria (PNH), and inherited bone marrow (BM) failure disorders are all considered BM failure states with significant clinicopathological overlap.1–3 However, the differentiation is critical as both AA and MDS-h have different management protocols with varying outcomes and prognoses. Compared to patients with AA, those with MDS-h have shorter median survival rates and exhibit a lower response to immunosuppressive therapy.4,5
AA is defined as pancytopenia in the peripheral blood secondary to BM hypocellularity and is a diagnosis of exclusion.6 MDS is a diverse collection of clonal disorders that affect the hematopoietic stem cells (HSCs) and are characterised by cytopenias of varying degrees in the peripheral blood and inefficient haematopoiesis with BM dysplasia. Myelodysplasias with hypocellular BM (MDS-h) comprises around 10–15% of all MDS cases and are characterized by BM hypocellularity. Both AA and MDS-h share a common pathophysiological pathway with CD34 positive progenitor cells being fundamental in the pathogenesis of both these entities. CD34 positive HSCs are the target of autoimmune attack in AA making its count significantly decreased in the BM of AA. Whereas CD34 progenitor cells are the cells from which MDS originates. This explains the elevated quantity of CD34 positive cells in the BM of patients with MDS as a result of neoplastic clonal expression.3
Recent studies have proposed an additional mechanism exploring the possibility of the role of BM niche in the pathogenesis of AA. The BM microenvironments also known as the stem cell niche comprises endosteal cells, macrophages, fat cells, fibroblasts, mast cells and microvascular endothelial cells.7 The stem cell microenvironment transmits signals to maintain the fundamental features of the HSCs such as the ability to self-regenerate and the capacity to reproduce all the lineages. Thus, alteration in the BM niche was considered as a potential cause of hematopoietic impairment; however, the ways in which these changes affect the development of the disease remains unclear.8
Several researchers have studied the correlation between the BM microenvironment and dysregulated haematopoiesis. Alterations in the components of the microenvironment in the BM, including lymphocytes, mast cells, macrophages, and stromal cells, may be associated with the impaired haematopoiesis leading to AA and/or myeloproliferative neoplasms. The quantitative increase of mast cells in AA cases has been demonstrated in several studies and has resulted in the hypothesis that mast cells can cause cytotoxic effects on the hematopoietic cells in the marrow.8
Mast cells originate from BM progenitor cells and migrate into various tissues to complete their maturation. BM mast cell quantification is proposed as a supplementary diagnostic and prognostic tool to differentiate AA from other BM failure conditions like MDS-h.5
The percentage of mast cells in the BM is generally less than 1% of all the nucleated cells in the marrow and is distributed singly.9 Mast cells stain metachromatically with Toluidine blue (TB), which stains the granules of mast cells purple to red. Histological staining with TB is a highly effective, rapid, direct and antibody-free technique to detect mast cells in tissue sections.10,11 Mast cells have longer life spans and are not directly targeted by the autoimmune attack on the stem cell compartment, resulting in the relative increase in its number in the BM of patients with AA. The quantitative increase in mast cell is clonal in mastocytosis and benign in acquired AA. However, its nature is not completely understood in AA and MDS-h.12,13
In AA, lower mast cell count is related to better prognosis, but no such relation exists in MDS.5 Several researchers have shown increased numbers of mast cells in cases of AA and have proposed an association between the increased mast cells in the BM with the poor outcome of the patients.14,15 Irrespective of the involved mechanism, a surge in the quantity of mast cells is assumed to be a factor responsible for the reduced cellularity observed in AA and MDS-h.16,17 Therefore, the present study aimed to evaluate the diagnostic utility of mast cell quantification in hypoplastic marrow (HM) to distinguish AA from MDS-h. Furthermore, the association of increased mast cells with decreased HSC in the BM of such entities was analysed.
All procedures performed in the current study were approved by Institutional Ethical Board, Kasturba Medical College, Mangalore (reference no.: IEC KMC MLR 12-2020/452 date: 24/12/2020). Formal written informed consent was not required since this study used retrospective biopsy samples and we received a waiver by the Institutional Ethical Board, Kasturba Medical College, Mangalore.
A time bound retrospective study was conducted at the Department of Pathology, Kasturba Medical College, Mangalore, MAHE, from 25th December 2020 to September 2022, after ethical clearance.
Cases of hypoplastic/aplastic BM biopsies received from January 2015 to December 2021 were included in the study. We calculated a minimum sample size of 58, considering 80% power, 95% confidence level and a relative precision of 10%. Overall, 65 cases met the inclusion criteria of adequate marrow biopsy measuring at least 1 cm in length with minimum four marrow spaces. Cases with measurable disease positive leukaemia, those with poorly preserved paraffin blocks and inadequate biopsies were excluded. Clinical data like age, sex and any other significant details were obtained from the laboratory information system and the medical records department. Our study follows the Sager guidelines for reporting sex and gender information. Sex and gender differences were not taken into consideration for the design of the study. The information regarding the sex of the cases was collected from the lab information system and not determined by the investigators as it is a retrospective study done on archived slides and blocks. The slides and paraffin blocks of all the cases were retrieved and reviewed and the diagnosis of hypoplasia/aplasia was confirmed.
The H&E-stained tissue sections were studied to assess the cellularity and to identify the morphology of the marrow. All the cases were categorised into three categories namely, AA, MDS-h and HM due to any other cause according to their cytomorphological and haematological features.
The special staining was done on paraffin embedded, formalin fixed tissue that was assessed for morphology. A representative block was selected in every case and TB special staining was performed, where 1% TB stain (1 gm TB in 100 ml H2O) was poured over the slides and kept for 90 seconds. The slides were then washed, dried and mounted.
Quantification of mast cells was done by identifying the metachromatic granules in mast cell cytoplasm. Total number of mast cells was counted per 200 nucleated cells in 5-10 randomly selected high power fields. Mast cell percentage amongst all nucleated cells was calculated. Mast cell percentage of more than one was quantified as increased.
Immunohistochemistry (IHC) for CD34 was performed on paraffin embedded, formalin fixed tissue that was assessed for morphology. A representative block was selected in every case. IHC staining was performed with a mouse monoclonal antibody against CD34 (Diagnostic BioSystems Cat# PDM050, RRID:AB_2934004). SITVue/DAB Detection System (Diagnostic BioSystems Cat# SIT-100D) was used as an intensification system to enhance the chromogenic signals of the primary antibody. The detailed description of all the procedural steps is available in Figshare.25 Evaluation for CD34 was done in the cytoplasm of the hematopoietic progenitor cells and the number of positive cells was counted and assessed as less than or more than 0.5% of the total hematopoietic cells.
Data were analysed using IBM SPSS Statistics (RRID:SCR_016479) 25.0 version. The statistical significance of all variables was calculated using the Chi-squared test and Fisher’s exact test. P<0.05 was taken to be significant.
This study included a total of 65 cases of hypoplastic and aplastic marrows that met the inclusion and exclusion criteria, of which 21 (32.3%) cases were AA, 12 cases were MDS-h (18.5%) and 32 (49.2%) cases were diagnosed as HM.
A bimodal peak was noticed in the age distribution pattern with the majority of the population being from the paediatric and adolescent age group (32.3%) and the second peak comprising of people above 60 years of age (28.7%). The rest of the cases belonged to the age group of 20-60 years old. Slight male predilection was noted with M: F ratio being 1.6:1. Overall, 38% (25) of the total population were female and 62% (40) of the population were male. The demographic data of the individual cases are available as Underlying data.25
Mast cells in the BM were highlighted by staining them with TB. Mast cells were counted amongst 200 nucleated cells. More than 1% of mast cells were seen in 36 (55.4%) of the total cases. Seven (10.8%) cases showed more than 10% of mast cells. Mast cell count of more than 1% was considered as increased. Increased numbers of mast cells were observed in most of the cases of AA (31, 48%) followed by HM (20, 31%), and MDS-h (14, 21%), while the rest of the cases showed less than 1% of mast cells in their marrow. The Chi squared test was done with respect to the increased number of mast cells and the different entities of AA, MDS-h and other causes of HM was significant (P: 0.040). The distribution of the increased mast cells amongst the various differentials in the contingency table is depicted in Table 1. Metachromatically stained mast cells in light microscopy in a case of AA and MDS-h are depicted in Figure 1 and Figure 2, respectively. These microphotographs are annotated and minimally cropped. The original unprocessed microphotographs are available as Underlying data.25
AA, aplastic anaemia; HM, hypoplastic marrow; MDS-h, hypocellular myelodysplastic neoplasm.
| Increased mast cells | AA | HM | MDS-h | |||
|---|---|---|---|---|---|---|
| Count, n | Percentage, % | Count, n | Percentage, % | Count, n | Percentage, % | |
| Absent | 5 | 23.8 | 16 | 50.0 | 8 | 66.7 |
| Present | 16 | 76.2 | 16 | 50.0 | 4 | 33.3 |
| Total | 21 | 100.0 | 32 | 100.0 | 12 | 100.0 |
| P value | 0.04 | |||||
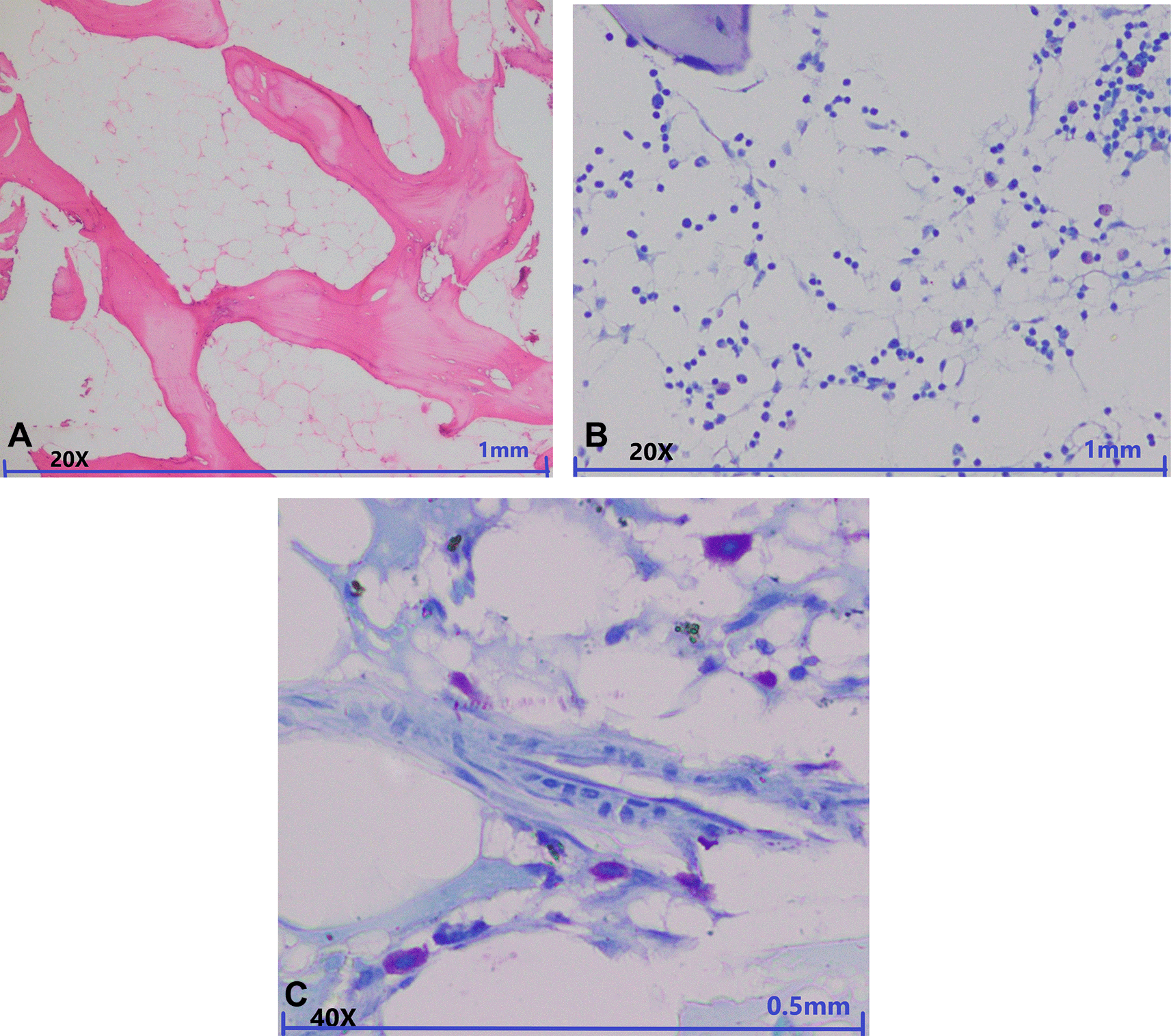
A: In low power view aplastic marrow is seen with cellularity less than 10% in the marrow spaces (magnification, 20×, H&E). B: Toluidine blue special staining in low power view shows metachromatically stained mast cells (magnification, 20×, Toluidine blue staining). C: Toluidine blue special staining in high power view shows six metachromatically stained mast cells in around 20-22 nucleated cells (magnification, 40×, Toluidine blue staining).
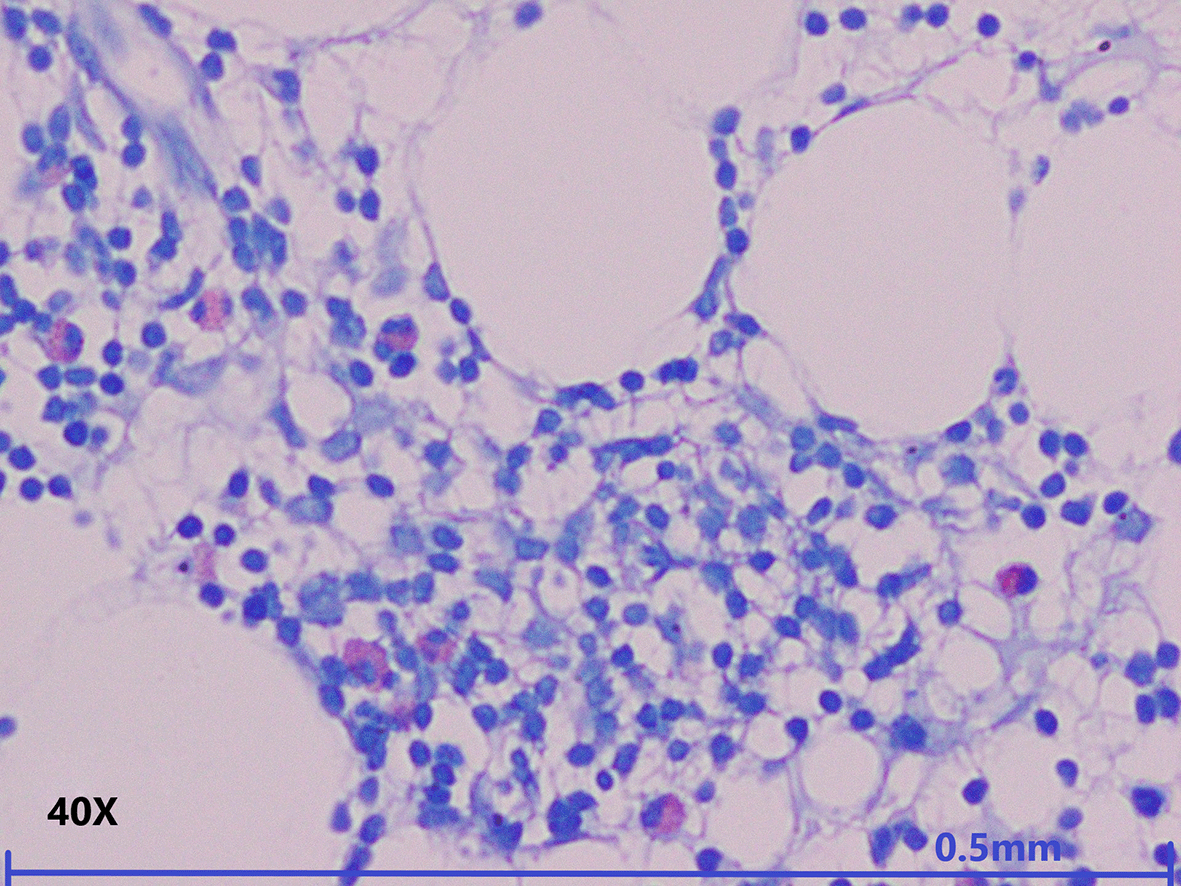
Toluidine blue special staining in high power view shows four metachromatically stained mast cells in around 200 nucleated cells (magnification, 40×, Toluidine blue staining).
CD34 IHC expression was assessed to calculate HSCs and categorised as more than and less than 0.5% of positive cells (decreased) amongst all the hematopoietic cells of the marrow. Decreased expression was noted in 45 (69.2%) of the cases.
An increased number of mast cells was seen in 36 (55.45%) cases of the total study population while significantly decreased/absent HSCs were seen in 45 (69.2%) cases. A total of 25 (69.4%) of the cases that showed an increased number of mast cells also showed significantly decreased HSCs. Whereas 26 (57.7%) of the cases that had decreased HSCs showed an increased number of mast cells. However, the association between decreased HSCs and increased mast cells was statistically insignificant (Chi squared test, P: 0.10). The contingency table of the same is depicted in Table 2.
BM failure conditions with marrow that is hypocellular for age embodies a broad spectrum of acquired and inherited conditions. Patients with MDS-h are known to have a worse prognosis than those with AA because they are more prone to neoplastic progression.18
Haematologists still continue to struggle in making a precise diagnosis due to the blurred lines between AA, other mimicking conditions like MDS-h and the various inherited BM failure syndromes. Identifying the exact disorder leading to a HM is critical as the cause of the disease significantly affects the choice of therapy.19,20
BM evaluation, comprising of both trephine biopsy and aspirate, is obligatory to ascertain the diagnosis. There is convincing evidence that these discrete haematological conditions share a conjoint pathophysiological pathway centred at the alteration and/or damage of the hematopoietic stem and progenitor cells (HSPCs) by the cytotoxic effect of T cells. Increased T cells produce excessive proinflammatory cytokines (interferon-γ and tumor necrosis factor-α), leading to the reduced proliferation and increased apoptosis of the HSPCs.21
The distinction between AA and MDS-h is mostly dependent on the histomorphological and IHC features of the BM but in HMs it is challenging due to the scarcity of the hematopoietic cells present in the BM and the overlapping of the cytomorphological features of both the entities.4 Erythroid dysplasia is not uncommon in AA and thus cannot be used as a sole distinguishing feature.
Mast cell quantification in the BM was performed by several researchers and a common finding of increased number of mast cells in HMs was observed.14–16,22 In our study we considered more than 1% of mast cells per 200 nucleated cells as the cut off for defining increase in the quantity of mast cells. The results in our study were similar to other studies with elevated mast cells seen in 55.4% of the total cases out of which the majority of the cases were AA (P<0.05). The quantity of mast cells ranged from 0-100 mast cells per 200 nucleated cells. The case with the highest number of mast cells belonged to the category of AA. However, the variation in the quantity of mast cells in both AA and MDS-h lies in the same range. This is consistent with the findings of Ingrid Fohlmeister22 done on 48 cases of different forms of MDS and 59 cases of AA. They demonstrated that there was overlap in the number of mast cells found in the BM of individuals with AA and MDS-h. The number of mast cells varied from 0-205/mm2.
Complex interactions between the hematopoietic cells and the BM niche occur during haematopoiesis, although the exact cause and mechanism involved in the deregulated haematopoiesis is unknown. HSCs reside in a specialized microenvironment (niche) in the BM. BM microenvironment is important in maintaining the function of HSC as it is thought to transmit signals sustaining key HSC properties like regenerating capability and multilineage reproducing capacity. Thus, alteration in the BM niche can be a potential factor associated with hematopoietic impairment. However, it is still unclear how these changes contribute to the development of the disease.
In cases of idiopathic AA, the ineffective haematopoiesis can be considered as a result of some unknown changes in the BM microenvironment. Mast cells are considered by some researchers as a cause of the ineffective haematopoiesis in AA as it stimulates apoptosis and auto immune attack by cytotoxic killer cells. Studies have demonstrated that the BM of patients with AA shows increases in the quantity of mast cells and natural killer (NK) cells reflecting the cytotoxic or immune-mediated damage of the marrow.8,11 Our study strengthens this hypothesis as 17 (48%) of the cases that showed an increased number of mast cells belonged to the patients with AA. Thus, increases in the numbers of mast cells can be considered as a causative factor for the insufficient haematopoiesis. However, in our study the association between increased mast cells and reduced HSCs was statistically insignificant.
Patients with AA may have altered BM niches that contribute to the disease pathophysiology or cause ineffective haematopoiesis. Quantification of mast cell can help in identifying the prognosis of the cases of AA. Reduced numbers of mast cells are a good prognostic indicator in AA while no such relation is observed in cases of MDS.16
Although hematopoietic stem cell transplantation plays a frontline role in the treatment of haemato-oncological conditions, there are still significant issues that need to be resolved, including the difficulty in finding matched donors and the ineffective engraftment of HSCs into the BM. An improved comprehension of the interactions amongst each component of the BM niche can help in identifying the potential therapeutic targets in cases of BM failure as well as myeloproliferative disorders.23
Simulating the hematopoietic niche is a promising method for effectively increasing the number of HSPCs and fine-tuning their features ex vivo. The creation of a functioning hematopoietic microenvironment in vitro is still constrained by attempts to replicate it without a thorough grasp of the specific roles played by individual components involved in the hematopoietic setting. Detailed knowledge of the crucial cells in the BM microenvironment and the way they affect the regulation of HSCs can aid in establishing an ex vivo structural arrangement for the expansion of HSCs to provide a novel source of therapeutic blood cells for haematological disorders.23,24
In conclusion, mast cell quantification in the BM biopsies with the help of TB special stain can act as a supplementary tool to distinguish the two entities as mast cells are increased in the majority of the cases of AA, while it is increased only in a few cases of MDS-h. The knowledge of the association between the increased number of mast cells with decreased HSCs can help in establishing an ex vivo environment mimicking the normal BM. This can aid in cultivating HSPCs for therapeutic purposes as well as in identifying the targets for targeted therapies in disorders like AA and other haematological malignancies.
Figshare: mast cell quantification raw data.xlsx. https://doi.org/10.6084/m9.figshare.21966848. 25
This project contains the following underlying data:
1. Excel sheet of complete data: rawdata.xlsx
2. STROBE_checklist. Mast cell quantification.docx
3. Unprocessed microphotographs: 1.jpeg, 2.jpeg, 3.jpeg, 4.jpeg, 5.jpeg, 6.jpeg
4. Procedure of CD34 IHC staining.docx
Data are available under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
References
1. Ribatti D, Polimeno G, Vacca A, Marzullo A, et al.: Correlation of bone marrow angiogenesis and mast cells with tryptase activity in myelodysplastic syndromes.Leukemia. 2002; 16 (9): 1680-4 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Bone marrow failure syndromes; flow cytometry immunophenotyping
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Hematologist
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 14 Apr 25 |
read | ||
|
Version 1 13 Jul 23 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)