Keywords
differentiation, epigenetics, eye development, single-cell ATAC-seq, single-cell RNA-seq
This article is included in the Cell & Molecular Biology gateway.
This article is included in the Japan Institutional Gateway gateway.
The regulation of receptor expression is crucial for fine-tuned signal transduction. Notch signaling is a key signaling pathway involved in retinal development. Compared to the knowledge on this signaling pathway in the differentiation of retinal ganglion cells, less is known about its involvement in earlier stages of retinal progenitor cell differentiation and its regulation, although NOTCH1, and probably NOTCH3, are involved in earlier stage differentiation. We aimed to clarify the timing of Notch receptor expression in undifferentiated retinal progenitor cells and elucidate the possible involvement of chromatin remodeling in the regulation of Notch receptor expressions.
We re-analyzed publicly available human fetal retina single-cell RNA-seq and ATAC-seq data (GSE183684) using Seurat/Signac pipelines.
On days 59, 74 and 78, we observed NOTCH1 and NOTCH3 mRNA expressions in early retinal progenitor cells, which diminished at later stages of differentiation. Integration of single-cell RNA-seq and ATAC-seq revealed that chromatin remodeling in part of the NOTCH1 and NOTCH3 loci were accompanied by transitions in their mRNA expressions. Importantly, the NOTCH1 locus, which showed chromatin remodeling, contained multiple binding motifs for transcription factors important in early retinal progenitor cell differentiation.
These results suggest that chromatin remodeling may be involved in the differential expression of NOTCH1, although another type of Notch mRNA expression regulations may also exist.
differentiation, epigenetics, eye development, single-cell ATAC-seq, single-cell RNA-seq
Thank you so much for reading our paper. We have made significant improvements as suggested by the two reviewers (Dr. Mariko Kashiwagi and Dr. Zhongjie Tang). Among the many improvements inspired by the reviewers’ insightful suggestions, the particularly important ones are broadening the genome region analysis (100 kb for both 5’ upstream and 3’ downstream regions of the gene encoding region), clarifying of the hierarchy of the four NOTCH receptor expressions, and analyzing the binding motifs for the key transcription factors (LHX2, PAX6, RAX and VSX2). The details of the changes in each section of the revised manuscript are described below.
In Abstract:
We have newly mentioned probable NOTCH3 involvement in retinal progenitor cell (RPC) differentiation. We have also included a summary of motif analyses for transcription factors important for early RPCs in chromatin remodeling regions in the NOTCH1 locus.
In Introduction:
We have included biological context of NOTCH3 in RPC differentiation for the purpose of clarifying the significance of NOTCH3 examination in this study.
In Methods:
We have described the detail of motif analyses for LHX2, PAX6, RAX and VSX2.
In Results:
For all the three data (day 59, 74 and 78), we have updated cell cluster names, expanded genomic ranges of chromatin accessibility analyses, and conducted the motif analyses. For visibility, we have separated the main figure for each day sample into two (e.g., Figure 1 and 2 for day 59). The details of cell clusters, chromosome regions of ATAC peaks, and motif analyses are supplementarily provided as csv or xlsx files. Moreover, we have significantly revised the text, corresponding to updated data and clarification of the hierarchy of observed NOTCH expressions.
In Discussion:
We have discussed epigenetic regulations of the four NOTCH genes, while addressing the discrepancy between mRNA expression and chromatin accessibility of NOTCH2 and NOTCH4.
See the authors' detailed response to the review by Mariko Kashiwagi
See the authors' detailed response to the review by Zhongjie Tang
Signal transduction depends on the expression of receptors regulated at multiple levels. These regulations include chromatin remodeling and DNA-binding proteins, such as transcription factors and transcriptional repressors. Because fine-tuned signal transduction is necessary for development, it is important to clarify the regulatory mechanisms of receptor expression for understanding the regulation of cell differentiation and subsequent tissue development.
Notch signaling in mammals is dependent on the binding of five canonical DSL ligands and four Notch receptors.1 It has been suggested that Notch loci are subject to chromatin remodeling under both normal2–7 and pathological8–16 conditions. In terms of developmental biology, the retina is a good model for investigating cellular differentiation involving Notch signaling.17 The developed retina is composed of multiple cell types, including retinal ganglion cells, bipolar cells, photoreceptors, amacrine cells, horizontal cells, and Müllar glial cells, all of which originate from the retinal progenitor cells (RPCs). RPC is characterized by the expression of Lhx2, Pax6, Rax and Vsx2 while there are some additional marker genes for developing horizontal cells/retinal ganglion cells (Onecut1/2),18 developing amacrine cells (Elavl2/4),19 developing photoreceptors/amacrine cells/Müllar glial cells (Eef1a1),20 developing retinal ganglion cells (Meis2),21 developing horizontal cells (Lhx1, Ptf1a),22 and glial cells (Pax2).23 Of the four Notch receptors in mammals, Notch1 has been suggested to be involved in the maintenance of RPCs and differentiation into retinal ganglion cells.24 In addition to Notch1, Notch3 expression has also been suggested in the early RPCs, which potentially affects the amount and oscillation of total Notch signaling via its susceptibility to the receptor cleavage and subsequent signal transduction.25 However, it is unclear when and how Notch receptor expression is switched on and off in RPCs during early differentiation. Here, we re-analyzed a public multi-omics dataset of single-cell RNA-seq and single-cell ATAC-seq from three human fetal retinas26 to clarify the timing of Notch receptor expression and examine the involvement of chromatin remodeling in this receptor expression switch.
A single-cell multi-omics dataset (GSE183684)26 was downloaded from the Gene Expression Omnibus (https://www.ncbi.nlm.nih.gov/geo/); data from days 59, 74 and 78 in the dataset were selectively used because they contained many undifferentiated retinal progenitor cells. The data were processed in Seurat version 5.1.027 and Signac version 1.14.028 pipelines in R version 4.4.1 on the Ubuntu 22.04.4 LTS environment. Pseudotime analysis and integration of the single-cell RNA-seq data and the single-cell ATAC-seq data was conducted by using the “FindTransferAnchors” function of Signac and Monocle3 version 1.3.7,29 respectively. The conditions used in the analysis are provided in the GitHub repository. We searched the transcription factor binding motifs for LHX2 (TAATTA),30 PAX6 (CGCCTGA),31,32 RAX ([C/T]AATTA)33,34 and VSX2 (TAATT [A/G])35 (“Supplementary_table_motif.xlsx”) manually by referencing human genome assembly hg38 in the University of California Santa Cruz genome browser (https://genome.ucsc.edu/).
First, early gestational stage samples were characterized (day 59). Uniform manifold approximation and projection (UMAP) analysis of single-cell RNA-seq identified 14 clusters (Figure 1A), which were further characterized by marker gene expressions (Figure 1B). This sample primarily contained RPCs with various differentiation statuses except for PAX2-expressing glial cells. We defined LHX2, PAX6, RAX and VSX2 as early markers. We considered that early markers-expressing RPCs and MKI67-expressing RPCs constitute early RPCs. In addition, pseudotime analysis suggested that RPCs decreased expressions of LHX2, PAX6, RAX and VSX2 during differentiation into either ONECUT1/ 2-, ELAVL2/4- or EEF1A1-expressing RPCs (Figure 1C). The details of each cluster are available in “Supplementary_table_count.xlsx” in our GitHub repository. Next, we examined Notch mRNA expressions and found that NOTCH1, NOTCH2 and NOTCH3 were expressed primarily in early RPCs with NOTCH1 showing the highest expression, which prompted us to focus on NOTCH1 and NOTCH3,25 whereas NOTCH4 expression was barely detectable (Figure 1D). Then, we re-analyzed single-cell ATAC-seq data, which was mathematically integrated with its single-cell RNA-seq data by using the “FindTransferAnchors” function of Signac (Figure 1E). In the early RPCs (Early markers-expressing RPC1- 3_d59 and MKI67-expressing RPC1- 2_d59), we observed multiple peaks in the 100 kb upstream and downstream chromosome regions of NOTCH1, some of which were diminished in the other RPC clusters such as ELAVL2/4- or EEF1A1-expressing RPCs (Figure 2A, arrowheads). Since these regions contained multiple binding motifs for LHX2, PAX6, RAX and VSX2 (Figure 2A, arrowheads numbered 1-3) (“Supplementary_table_motif.xlsx”), chromatin remodeling of these regions possibly regulates NOTCH1 expression transition during RPC differentiation although the expression transitions of LHX2, PAX6, RAX and VSX2 may also support the NOTCH1 expression transition (Figure 2A). Since NOTCH3 has also been suggested for early RPC differentiation,25 we also conducted chromatin accessibility analysis of NOTCH3 and observed less prominent chromatin remodeling in the 100 kb upstream and downstream regions spanning the NOTCH3 locus and the mRNA diminishment supports the previous report25 (Figure 2B). The tables of the chromosome regions of each variable peak in NOTCH1 and NOTCH3 are provided in our GitHub repository as “ATAC_peaks_NOTCH1_d59.csv” and “ATAC_peaks_NOTCH3_d59.csv”, respectively. Although NOTCH2 and NOTCH4 have been less documented for RPC differentiation in the literature, we have provided the dot plots of the Notch expressions and the coverage plots of NOTCH2 and NOTCH4 in “Additional_file_1.tif” in our GitHub repository. In contrast to NOTCH2 mRNA expression in the early RPCs (Figure 1D), we observed several peaks in the chromosome region spanning the NOTCH2 locus although it was difficult to point out the peak regions specific for the early RPCs (Additional_file_1C). Conversely, in contrast to little NOTCH4 mRNA expression (Figure 1D), we found that multiple peaks were constantly open in the NOTCH4 locus during RPC differentiation (Additional_file_1D, arrowheads), some of which contained multiple binding motifs for LHX2, PAX6, RAX and VSX2 (Additional_file_1D, arrowheads numbered 1-2) (“Supplementary_table_motif.xlsx”). The table of the chromosome regions of each variable peak in NOTCH4 is provided in our GitHub repository as “ATAC_peaks_NOTCH4_d59.csv.”
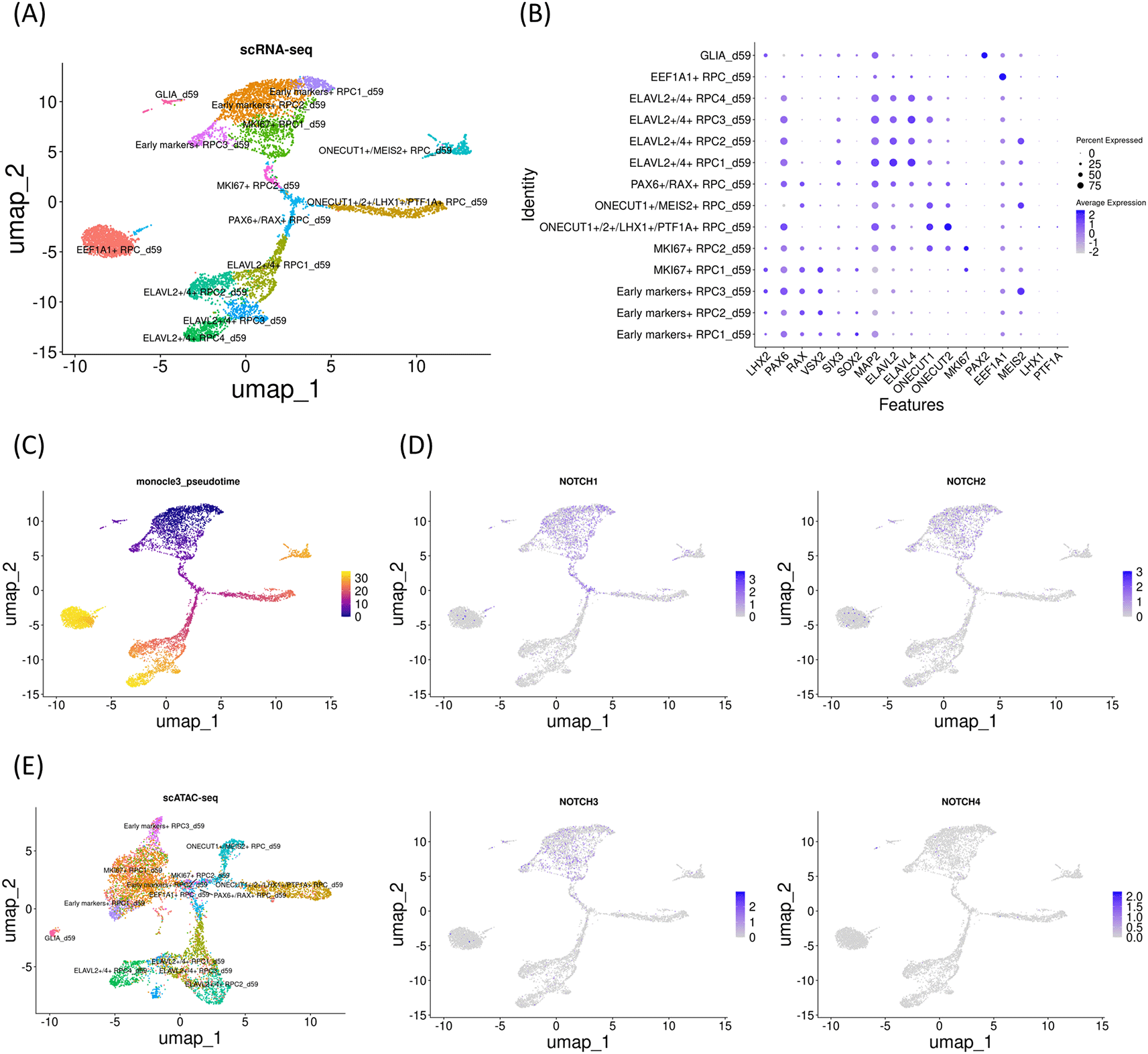
UMAP analysis of the single-cell RNA-seq data identified 14 clusters. (B) Dot plot of marker genes. (C) Monocle3 pseudotime analysis for clarifying the differentiation status. (D) Feature plot of NOTCH1-4. Note that NOTCH1, NOTCH2 and NOTCH3 expressions were prominent in the early RPC clusters. (E) UMAP analysis of the single-cell ATAC-seq data, which was integrated with the single-cell RNA-seq data.
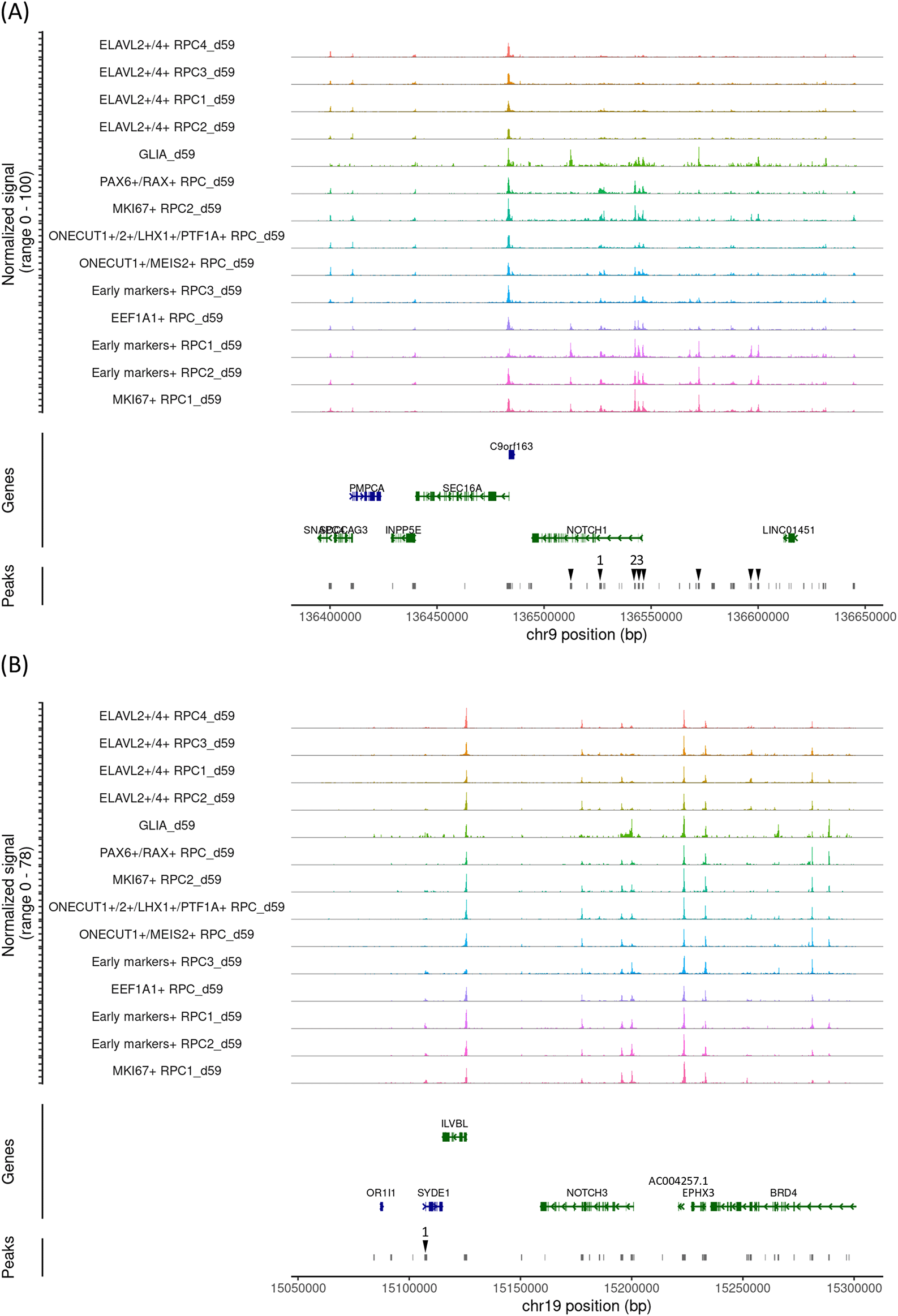
The coverage plot of the 100 kb upstream and downstream chromosome regions of NOTCH1. The upstream of the genes is on the right. Variable peak regions are indicated by arrowheads, with the regions containing transcription binding motifs numbered as 1-3. (B) The coverage plot of the 100 kb upstream and downstream chromosome regions of NOTCH3. The upstream of the genes is on the right. A variable peak region which contains transcription binding motifs is indicated by an arrowhead numbered as 1.
To validate the changes in Notch mRNA expressions and chromatin accessibility in the Notch loci during RPC differentiation, we investigated another early gestational stage sample (day 74). UMAP analysis identified 15 clusters (Figure 3A) that were further characterized by marker gene expressions (Figure 3B). This sample contained RPCs with various differentiation statuses. Similar to day 59 sample, pseudotime analysis suggested that the early RPCs differentiated into either ELAVL2/4, ONECUT1/MEIS2 or ONECUT1/2-expressing RPCs (Figure 3C). ONECUT1/MEIS2-expressing RPCs differentiated into RPCs that markedly expressed VSX2. Next, we examined Notch mRNA expressions and found that NOTCH1, NOTCH2 and NOTCH3 were expressed primarily in early RPCs with NOTCH1 showing the highest expression whereas NOTCH4 expression was barely detectable (Figure 3D), similar to the day 59 sample. We then re-analyzed the single-cell ATAC-seq data, which were integrated with the single-cell RNA-seq data (Figure 3E). In the early RPCs (Early markers-expressing RPC1- 4_d74 and MKI67-expressing RPC_d74), we observed multiple peaks in the 100 kb upstream and downstream chromosome regions of NOTCH1, some of which were diminished in the other RPC clusters, such as ELAVL2/4- or ONECUT1/2-expressing RPCs (Figure 4A, arrowheads). Since these regions contained multiple binding motifs for LHX2, PAX6, RAX and VSX2 (Figure 4A, arrowheads numbered 1-4) (“Supplementary_table_motif.xlsx”), chromatin remodeling of these regions possibly regulates NOTCH1 expression transition during RPC differentiation although the expression transitions of LHX2, PAX6, RAX and VSX2 may also support the NOTCH1 expression transition (Figure 4A). Since NOTCH3 has also been suggested for early RPC differentiation,25 we also conducted chromatin accessibility analysis of NOTCH3 and observed less prominent chromatin remodeling in the 100 kb upstream and downstream regions spanning the NOTCH3 locus (Figure 4B). The tables of the chromosome regions of each variable peak in NOTCH1 and NOTCH3 are provided in our GitHub repository as “ATAC_peaks_NOTCH1_d74.csv” and “ATAC_peaks_NOTCH3_d74.csv”, respectively. In summary, similar to the day 59 sample, in the day 74 sample, a concomitant mRNA decrease and chromatin remodeling in the NOTCH1 locus were observed. Although NOTCH2 and NOTCH4 have been less documented for RPC differentiation in the literature, we have provided the dot plots of the Notch expressions and the coverage plots of NOTCH2 and NOTCH4 in “Additional_file_2.tif” in our GitHub repository. In contrast to NOTCH2 mRNA expression (Figure 4D), we observed a single peak in the NOTCH2 locus for EEF1A1-expressing RPC_d74, and therefore, no peak regions in the NOTCH2 locus specific for the early RPCs (Additional_file_2C). Converesly, in contrast to little NOTCH4 mRNA expression (Figure 4D), we found that multiple peaks were constantly open in the NOTCH4 locus during RPC differentiation (Additional_file_2D, arrowheads), some of which contained multiple binding motifs for LHX2, PAX6, RAX and VSX2 (Additional_file_2D, arrowheads numbered 1-3) (“Supplementary_table_motif.xlsx”). The table of the chromosome regions of each variable peak in NOTCH4 is provided in our GitHub repository as “ATAC_peaks_NOTCH4_d74.csv.”
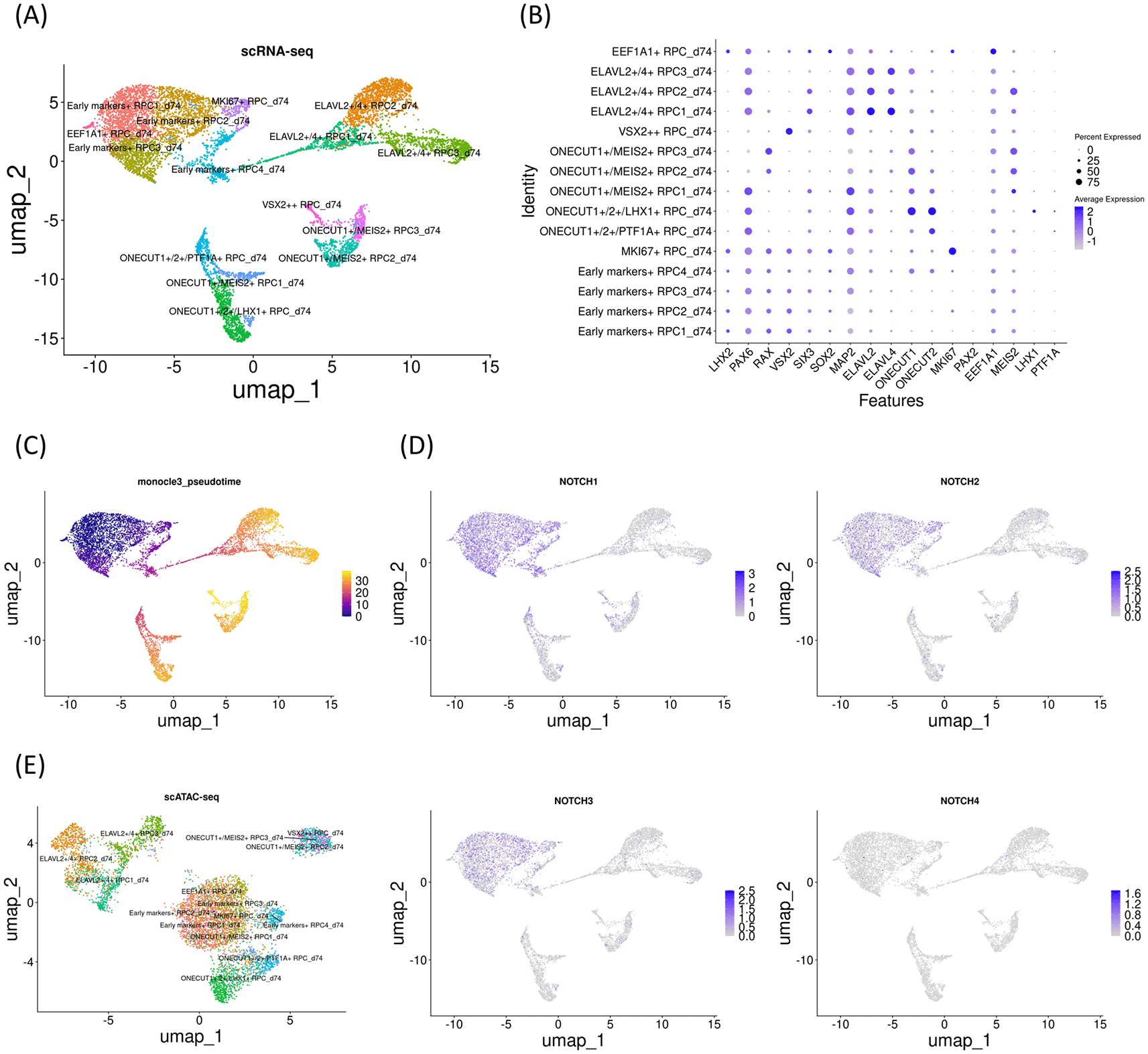
(A) UMAP analysis of the single-cell RNA-seq data identified 15 clusters. (B) Dot plot of marker genes. (C) Monocle3 pseudotime analysis. (D) Feature plot of NOTCH1-4. Note that NOTCH1, NOTCH2 and NOTCH3 expressions were prominent in the early RPC clusters. (E) UMAP analysis of the single-cell ATAC-seq data which was integrated with the single-cell RNA-seq data.
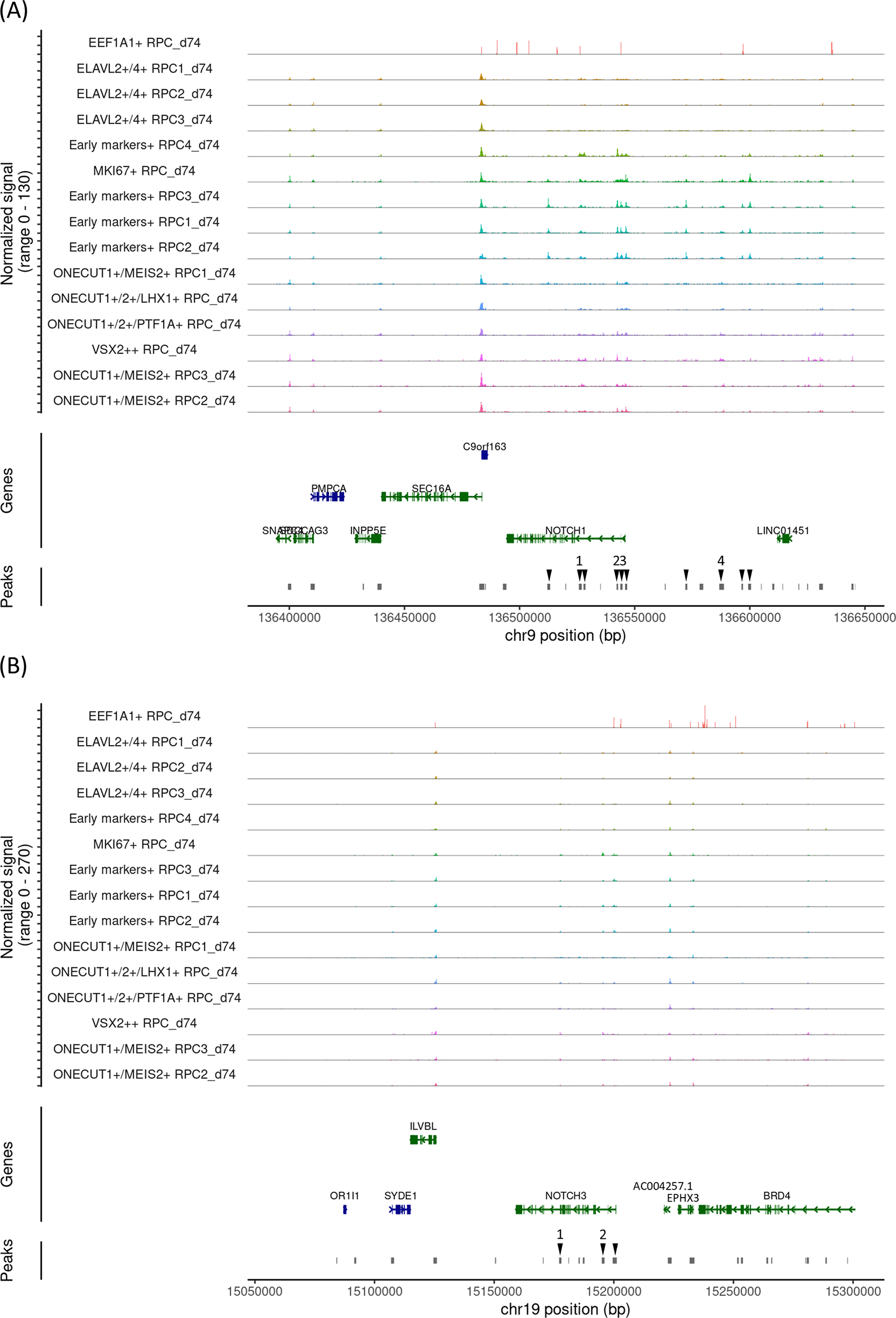
(A) The coverage plot of the 100 kb upstream and downstream chromosome regions of NOTCH1. The upstream of the gene is on the right. Variable peak regions are indicated by arrowheads, with the regions containing transcription binding motifs numbered as 1-4. (B) The coverage plot of the 100 kb upstream and downstream chromosome regions of NOTCH3. The upstream of the gene is on the right. Variable peak regions are indicated by arrowheads, with the regions containing transcription binding motifs numbered as 1-2.
To further confirm the developmental changes in Notch mRNA expression and chromatin accessibility at the Notch loci, we added one more early gestational stage sample (day 78). UMAP analysis identified 18 clusters (Figure 5A) that were further characterized by marker gene expressions (Figure 5B). The sample contained RPCs with various differentiation statuses. Pseudotime analysis suggested that the early RPCs differentiated into either ONECUT1/2-expressing RPCs, which later differentiated into PTF1A and LHX1-expressing RPCs, ONECUT1/MEIS2-expressing RPCs, or ELAVL2/4-expressing RPCs (Figure 5C). Next, we examined Notch mRNA expressions and found that NOTCH1, NOTCH2 and NOTCH3 were expressed primarily in early RPCs with NOTCH1 showing the highest expression whereas NOTCH4 expression was barely detectable (Figure 5D), similar to the day 59 and 74 samples. We then re-analyzed the single-cell ATAC-seq data, which was integrated with the single-cell RNA-seq data (Figure 5E). In the early RPCs (Early markers-expressing RPC1-3_d78 and MKI67-expressing RPC1-2_78), we observed multiple peaks in the 100 kb upstream and downstream chromosome regions of NOTCH1, some of which were diminished in the other RPC clusters (Figure 6A, arrowheads). Since these regions contained multiple binding motifs for LHX2, PAX6, RAX and VSX2 (Figure 6A, arrowheads numbered 1-4) (“Supplementary_table_motif.xlsx”), chromatin remodeling of these regions possibly regulates NOTCH1 expression transition during RPC differentiation although the expression transitions of LHX2, PAX6, RAX and VSX2 may also support the NOTCH1 expression transition (Figure 6A). Since NOTCH3 has also been suggested for early RPC differentiation,25 we also conducted chromatin accessibility analysis of NOTCH3 and observed less prominent chromatin remodeling in the 100 kb upstream and downstream regions spanning the NOTCH3 locus (Figure 6B). The tables of the chromosome regions of each variable peak in NOTCH1 and NOTCH3 are provided in our GitHub repository as “ATAC_peaks_NOTCH1_d78.csv” and “ATAC_peaks_NOTCH3_d78.csv”, respectively. In summary, examinations of all the three independent samples suggested that NOTCH1 mRNA expression decreased as RPC differentiation progresses, which was concomitant with chromatin remodeling in the NOTCH1 locus. Importantly, chromatin remodeling regions contained multiple transcription factor binding motifs. Although NOTCH2 and NOTCH4 have been less documented for RPC differentiation in the literature, we have provided the dot plots of the Notch expressions and the coverage plots of NOTCH2 and NOTCH4 in “Additional_file_3.tif” in our GitHub repository. In contrast to NOTCH2 mRNA expression (Figure 5D), we observed several peaks in the chromosome regions spanning the NOTCH2 locus although it was difficult to point out peak regions specific for the early RPCs (Additional_file_3C). Conversly, in contrast to little NOTCH4 mRNA expression (Figure 5D), we found that multiple peaks were constantly open in the NOTCH4 locus during RPC differentiation (Additional_file_3D, arrowheads), some of which contained multiple binding motifs for LHX2, PAX6, RAX and VSX2 (Additional_file_3D, arrowheads numbered 1-3) (“Supplementary_table_motif.xlsx”). The table of the chromosome regions of each variable peak in NOTCH4 is provided in our GitHub repository as “ATAC_peaks_NOTCH4_d78.csv.”
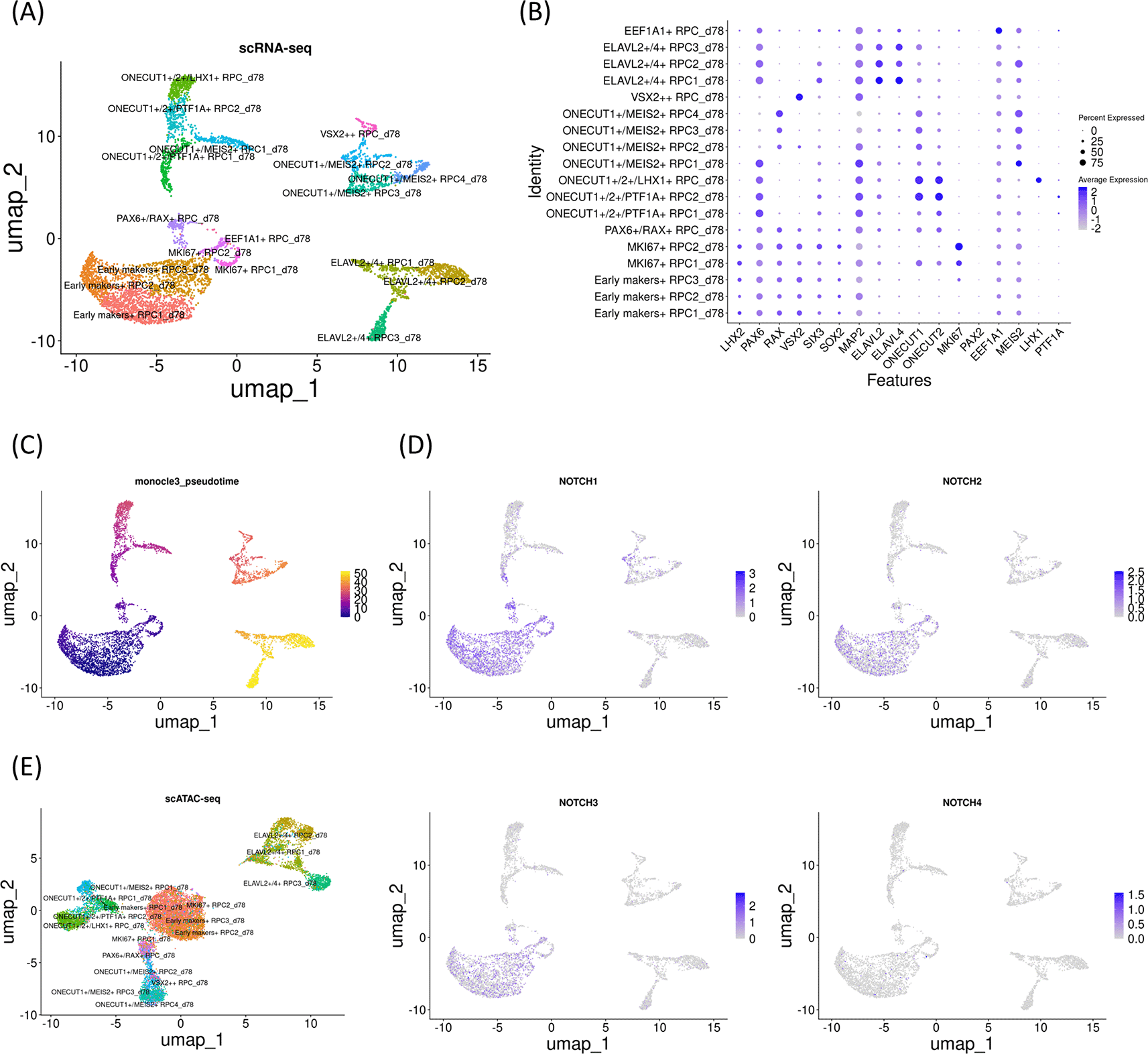
(A) UMAP analysis of the single-cell RNA-seq data identified 18 clusters. (B) Dot plot of marker genes. (C) Monocle3 pseudotime analysis. (D) Feature plot of NOTCH1- 4. Note that NOTCH1, NOTCH2 and NOTCH3 expressions were prominent in early RPC clusters. (E) UMAP analysis of the single-cell ATAC-seq data which was integrated with the single-cell RNA-seq data.
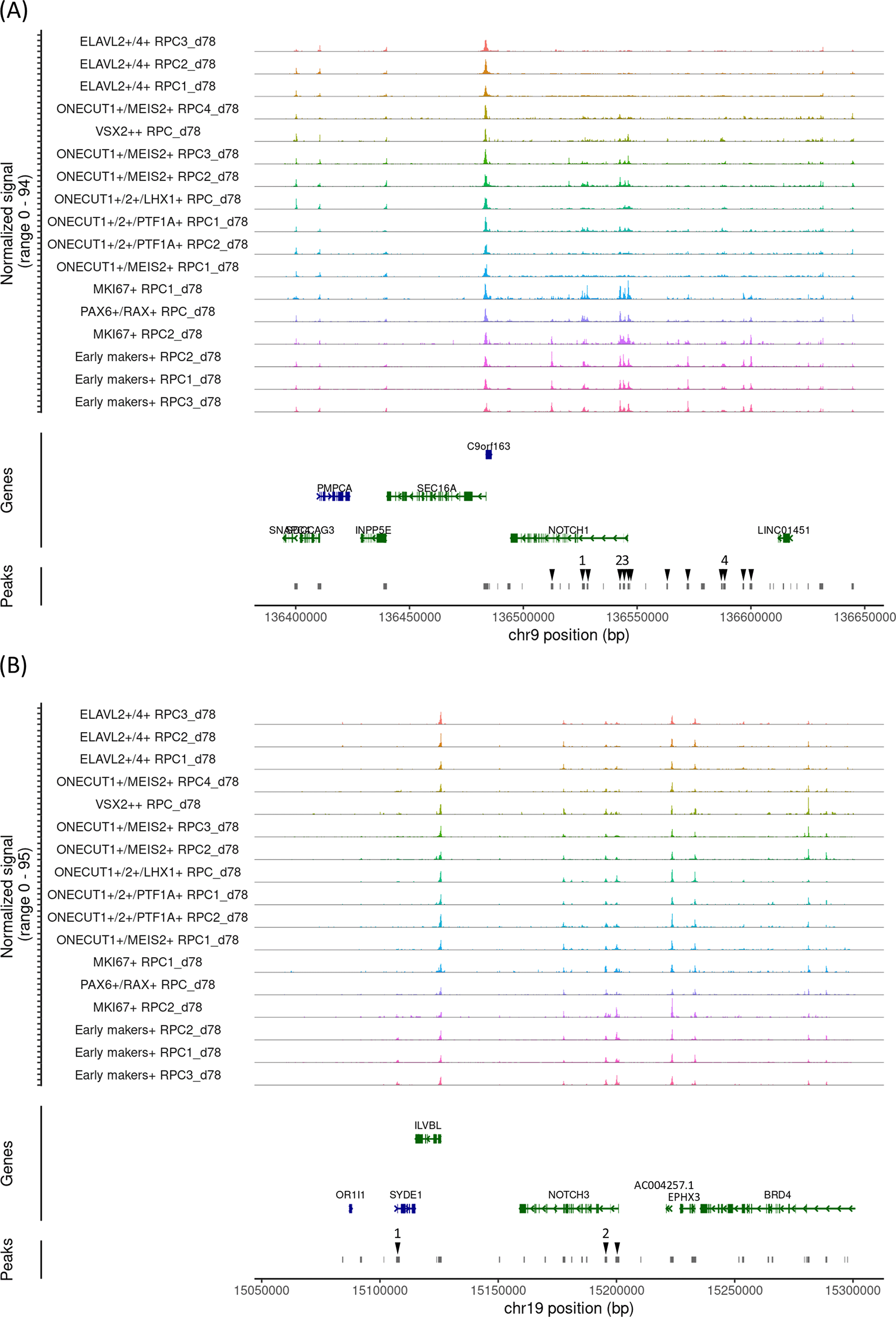
(A) The coverage plot of the 100 kb upstream and downstream chromosome regions of NOTCH1. The upstream of the gene is on the right. Variable peak regions are indicated by arrowheads, with the regions containing transcription binding motifs numbered as 1-4. (B) The coverage plot of the 100 kb upstream and downstream chromosome regions of NOTCH3. The upstream of the gene is on the right. Variable peak regions are indicated by arrowheads, with the regions with transcription binding motifs are numbered as 1-2.
The involvement of Notch signaling in cell fate choices is well documented, including in Drosophila neurogenesis36 and mammalian biliary development.37 Although the regulation of Notch receptor expression is necessary for these processes, to the best of our knowledge, few studies have used genome-wide investigations of the underlying molecular mechanisms. To examine chromatin remodeling in such regulatory mechanisms, we re-analyzed a single-cell RNA-seq and ATAC-seq dataset from developing retinas in which differentiation trajectories were well characterized. By re-analyzing three independent samples, we observed chromatin remodeling in the NOTCH1 locus, which contains multiple binding motifs for transcription factors, concomitant with changes in its mRNA expression during RPC differentiation. These findings highlight that the fine-tuned regulation of Notch receptor expressions occurs at an epigenetic level.
NOTCH1 and NOTCH3 were well documented in the early RPC differentiation compared to NOTCH2 and NOTCH4. Indeed, we observed NOTCH1 and NOTCH3 mRNA expressions in contrast to little NOTCH4 mRNA expression throughout the three samples. We observed comparable NOTCH2 mRNA expression in the early RPCs although we are unable to fully explain the discrepancy between mRNA expression and chromatin accessibility regarding this gene owing to lack of significant peak regions in the NOTCH2 locus (Additional_file_1C, 2C and 3C). Some possible causes such as regulation via distal enhancer and technical limitations of ATAC-seq may exist. When NOTCH2 mRNA is transcribed in support with those mechanisms, the decrease of NOTCH2 mRNA expression during RPC differentiation may be influenced by a post-transcriptional mechanism since a small non-coding regulatory RNA (CAT1) reportedly promotes stabilization of Notch2 mRNA.38 The roles and regulations of NOTCH2 are, however, beyond the scope of this study. Conversely, we observed little NOTCH4 mRNA expression and its high chromatin accessibility with multiple binding motifs for LHX2, PAX6, RAX and VSX2. This observation might be owing to the absence of other transcription factors for NOTCH4 mRNA expression since this gene expression has been suggested to highly depend on the cell type and its expressing transcription factors. For example, Notch4 is limitedly expressed in endothelial cells, compared to Notch1, under regulation of AP-1 transcription factor.39 Although we are unable to provide solid conclusions on the expression and regulation of NOTCH2 and NOTCH4 in the early RPCs, we believe that this does not impede our findings that NOTCH1 mRNA expression transition during RPC differentiation accompanied chromatin remodeling.
An ophthalmological study revealed that the epigenetic landscape of cell type-specific enhancers shifted during differentiation of RPCs.40 For example, in the single-cell ATAC-seq data from embryonic day 14.5 mouse retina, motif enrichment for Lhx2, Rax and Pax6 in the early RPCs were observed, and footprinting analysis validated binding of those transcription factors to their motifs. These high chromatin accessibilities decreased as they differentiated into retinal ganglion cells and non-retinal ganglion cells. Although that study is excellent in providing comprehensive and in-depth insights, ours is unique in focusing on the Notch loci for clarifying the regulatory mechanisms in view of Notch signaling biology.
Finally, we note that further investigations, such as a large deletion of these regions, will be needed to evaluate the contribution of the identified chromatin remodeling to the differential expression of Notch receptors in RPC subsets.
Y. W.: Data Curation, Formal Analysis, Software, Visualization, Writing – Original Draft Preparation
S.K.: Writing – Original Draft Preparation
T.N.: Writing – Original Draft Preparation
S.T.: Supervision, Writing – Review & Editing
M.Y.: Conceptualization, Formal Analysis, Funding Acquisition, Methodology, Project Administration, Software, Writing – Original Draft Preparation
A single-cell multiomics dataset (GSE183684)26 was downloaded from the Gene Expression Omnibus database (https://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE183684).
The original data in the present study including additional data available from: https://github.com/Yoshitokky/ eyeATAC_NOTCH1_data/tree/main.41
Archived software available from: https://doi.org/10.5281/zenodo.17084024.
License: OSI approved open license software is under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The project contains the following underlying data:
• Figure_1A.png
• Figure_1B.png
• Figure_1C.png
• Figure_1D_NOTCH1.png
• Figure_1D_NOTCH2.png
• Figure_1D_NOTCH3.png
• Figure_1D_NOTCH4.png
• Figure_1E.png
• Figure_2A.png
• Figure_2B.png
• Figure_3A.png
• Figure_3B.png
• Figure_3C.png
• Figure_3D_NOTCH1.png
• Figure_3D_NOTCH2.png
• Figure_3D_NOTCH3.png
• Figure_3D_NOTCH4.png
• Figure_3E.png
• Figure_4A.png
• Figure_4B.png
• Figure_5A.png
• Figure_5B.png
• Figure_5C.png
• Figure_5D_NOTCH1.png
• Figure_5D_NOTCH2.png
• Figure_5D_NOTCH3.png
• Figure_5D_NOTCH4.png
• Figure_5E.png
• Figure_6A.png
• Figure_6B.png
• Additional_file_1.tif (Extended data for d59 data).
• Additional_file_2.tif (Extended data for d74 data).
• Additional_file_3.tif (Extended data for d78 data).
• Additional_file_1_A.png
• Additional_file_1_B.png
• Additional_file_1_C.png
• Additional_file_1_D.png
• Additional_file_2_A.png
• Additional_file_2_B.png
• Additional_file_2_C.png
• Additional_file_2_D.png
• Additional_file_3_A.png
• Additional_file_3_B.png
• Additional_file_3_C.png
• Additional_file_3_D.png
• Supplementary_table_count.xlsx
• Supplementary_table_motif.xlsx
• ATAC_peaks_NOTCH1_d59.csv
• ATAC_peaks_NOTCH3_d59.csv
• ATAC_peaks_NOTCH4_d59.csv
• ATAC_peaks_NOTCH1_d74.csv
• ATAC_peaks_NOTCH3_d74.csv
• ATAC_peaks_NOTCH4_d74.csv
• ATAC_peaks_NOTCH1_d78.csv
• ATAC_peaks_NOTCH3_d78.csv
• ATAC_peaks_NOTCH4_d78.csv
Source code available from: https://github.com/Yoshitokky/eyeATAC_NOTCH1_software/tree/main.42
Archived software available from: https://doi.org/10.5281/zenodo.17084033.
License: OSI approved open license software is under the terms of the Creative Commons Zero “No rights reserved” data waiver (CC0 1.0 Public domain dedication).
The project contains the following underlying data:
• 250907_retina_chromatin_d59_No0.R (R code script for d59 data followed by No1).
• 250907_retina_chromatin_d59_No1.R (R code script for d59 data followed by No2).
• 250907_retina_chromatin_d59_No2.R (R code script for d59 data followed by No3).
• 250907_retina_chromatin_d59_No3.R (R code script for d59 data followed by No4).
• 250907_retina_chromatin_d59_No4.R (R code script for d59 data).
• 250907_retina_chromatin_d74_No0.R (R code script for d74 data followed by No1).
• 250907_retina_chromatin_d74_No1.R (R code script for d74 data followed by No2).
• 250907_retina_chromatin_d74_No2.R (R code script for d74 data followed by No3).
• 250907_retina_chromatin_d74_No3.R (R code script for d74 data followed by No4).
• 250907_retina_chromatin_d74_No4.R (R code script for d74 data).
• 250907_retina_chromatin_d78_No0.R (R code script for d78 data followed by No1).
• 250907_retina_chromatin_d78_No1.R (R code script for d78 data followed by No2).
• 250907_retina_chromatin_d78_No2.R (R code script for d78 data followed by No3).
• 250907_retina_chromatin_d78_No3.R (R code script for d78 data followed by No4).
• 250907_retina_chromatin_d78_No4.R (R code script for d78 data).
The authors thank Editage (https://www.editage.jp) for their support with English language editing.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Single-cell RNA-seq and ATAC-seq analysis, computational biology
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Single-cell RNA-seq and ATAC-seq analysis, computational biology
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Skin biology, Immunology, Gene regulation
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 14 Nov 25 |
||
|
Version 2 (revision) 22 Sep 25 |
read | |
|
Version 1 06 Jan 25 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)