Keywords
GLUT1 glucose transporter deficiency syndrome, Human glucose transporters, SLC transporter family, transport mechanism, molecular dynamics simulation, Martini force field, coarse-grained simulations, enhanced sampling method
This article is included in the Cell & Molecular Biology gateway.
This article is included in the Bioinformatics gateway.
GLUT1 glucose transporter deficiency syndrome, Human glucose transporters, SLC transporter family, transport mechanism, molecular dynamics simulation, Martini force field, coarse-grained simulations, enhanced sampling method
In the revised version we have clarified the scope of the study, which aims to study the impact of mutations on the first part of the transporter mechanism without considering the impact of glucose or other ligands. We have renamed our method to ComDYN for „COMmon constraints DYNamics“ as retaining only common constraints in the elastic network is the key ingredient for the enhanced conformational sampling. To study the complete pathway and elucidate the effect of all mutations in future studies, the simulations need to be extended towards the outward-open conformation, and also consider the impact of the ligand upon the transportation mechanism.
We would like to thank the reviewers again for providing us with useful comments and suggestions which helped improve our manuscript.
See the authors' detailed response to the review by Jocelyne Vreede
See the authors' detailed response to the review by Lucie Delemotte
The solute carrier (SLC) transporter superfamily is known to play a key role in the transport of small molecules. The superfamily comprises 52 families, and at least 386 different transporter genes have so far been identified in humans (Hediger et al., 2013; Higuchi et al., 2018). This family of membrane proteins is a large class of transporters for many small molecules such as glucose that are vital for the cell, and can be found in all kingdoms of life. Of particular interest are the glucose transporters SLC2A1 from the SLC2 subfamily; GLUT1 mutations are associated with GLUT1 deficiency syndrome (GLUT1DS1 and GLUT1DS2), and some forms of spasticity (Dystonia-9) and epilepsy (EIG2) (Klepper et al., 2016; Mongin et al., 2016). Shedding light on the molecular mechanism of the transport function, enables us to understand the difference between pathogenic and benign mutations that have been observed in human subjects. Glucose transporter GLUT1 is built from 12 transmembrane helices (TMs) and exhibits a two-fold symmetry plane joining the two times six TM helices over a bridging helix on the cytoplasmic side of the membrane (see Figure 1A). The transport mechanism of glucose involves cycling through four states (Deng et al., 2015): outward open (ligand bound or ligand free), outward occluded (OO, ligand bound), inward open (IO, ligand bound or ligand free), and inward occluded (ligand free), as summarized in Figure 1B. Throughout the SLC transporters, a highly conserved RXGRR-motif is found between TM2 and TM3 and between TM8 and TM9 at the intracellular side of the corresponding loops (Pao et al., 1998; Sato & Mueckler, 1999). Several mutations at these anchor points are known to be disease-related, such as G91D and R92W which are known to cause GLUT1DS1 (Klepper et al., 2001; Klepper & Voit, 2002), whereas R93W is associated with GLUT1DS2 (Joshi et al., 2008). Between TM8 and TM9, R333W is also confirmed to be pathogenic (Klepper et al., 2001; Klepper & Voit, 2002), while the clinical significance of R334Q is unknown, although it is likely to affect the protein function.

(A) Pipe representation of the inward-open (IO) conformation (PDB-ID: 5EQI; bound inhibitor removed) of GLUT1 situated in the lipid bilayer. Note that the protein structure has a two-fold rotational symmetry and the two conserved RXGRR-motifs are located at the junctions of the transmembrane (TM) helices 2 and 3 and TM8-TM9, around the R333/R334 and G91/R92 mutation sites shown in magenta. The red arrows symbolize the inside and outside distances. Note that we number the helices starting from TM1 at the N-terminus of the transporter (dark blue in the pipe representation). (B) Schematic cycle of the glucose transport mechanism between the four different open and occluded states as adapted from (Deng et al., 2015). The bound glucose ligand is indicated as a red sphere. In the open states (inward or outward), glucose may be bound or unbound; this is represented using white dots. This work concerns the first part of the transport mechanism, i.e., the dynamics between the outward-occluded state (OO) and inward-open state (IO) as highlighted with red arrows. Note however, that we do not consider ligand binding within the scope of this work (see Discussion further below). (C) Pipe representation of the IO conformation (PDB-ID: 5EQI) of GLUT1 viewing on the outward facing part of the transporter inside the periplasm. (D) Definition of the order parameters to follow the motion of the helices over the ComDYN simulations.
Here, we investigate the effect of different mutations on the dynamic of the human GLUT1 protein. As the dynamic response upon mutation may depend on the conformational state, we aimed to simulate the first part of the transport mechanism between the outward-occluded state (OO) and inward-open state (IO), as shown in Figure 1B. Hereby, we investigate the intrinsic dynamics of the transporter protein without the ligand. For GLUT1, however, a three-dimensional structure is only available for the IO state; therefore, simulations of GLUT3, which is evolutionarily quite close to GLUT1, in the OO state were analysed. GLUT3 is also suspected to be associated with neurological disorders such as Alzheimer (An et al., 2018; Gu et al., 2018; Simpson et al., 1994; Szablewski, 2017). We expect that the intrinsic dynamics of the ligand-free states of the transporter provides new insight on the effects of the pathogenicity of certain mutations, without explicit consideration of the ligand-bound states.
The crystal structures of both IO and OO states are available in the PDB; 5EQI for GLUT1 IO state (Deng et al., 2015), and 4ZW9 for GLUT3 OO state (Kapoor et al., 2016); these were used as starting points for the simulations, as we focus on the first part of the transport mechanism. The next step, i.e., to elucidate the complete pathway, would be to extend our work to the outward-open conformation which is also available in the PDB (4ZWC GLUT3 with maltose bound), and to study the effect of ligand binding on the transporter dynamics. However, this is beyond the scope of the present study. Based on the reported pathogenic mutations of GLUT1 in the conserved RXGRR motif region, we searched the corresponding positions of GLUT3 for additional mutations and included them in our study. Due to the high sequence identity (~70%) between the proteins, we intentionally did not build a homology model for one or the other protein. This is justified, as our main aim is to characterize the global opening and closing mechanism rather than to look into atomistic details such as protonation states. Moreover, we now avoid additional uncertainties about details of the structure as would be inevitably introduced during the homology modelling process. Our composite scheme using coarse-grained molecular dynamics with common constraints elastic network ‘ComDYN’, that keeps part of the unchanged position constraints between the two protein structures, is explained in the approach below and further details are supplied in the supporting methods, available in the deposited code (Feenstra, 2019b).
In this study, we employed molecular dynamics (MD) simulations using the GROMACS 4.0.5 programme package (Hess et al., 2008). For efficiency reason, we investigated the applicability of the MARTINI coarse-grained (CG) force field (Arnarez et al., 2015; de Jong et al., 2013; Hsu et al., 2017; Monticelli et al., 2008; Periole et al., 2009), which is about 500-fold faster than the full-atomistic GROMOS (May et al., 2014). This speed-up is obtained at the expense of explicit description of hydrogen bonds in the protein, which necessitates the addition of an elastic network to maintain secondary structure and other tertiary contacts and thus overall protein stability; the elastic network is thus tailed to a particular conformational state of the protein. Here, we propose to modify the elastic network that is used in MARTINI based on our starting conformations, to include only common elastic network constraints that differed less than 1Å between the IO and OO states. Increasing the distance threshold for the common constraints would result in even fewer constraints in the ComDYN, increasing the risk of instability and possibly unfolding of the protein. Therefore, our chosen constraints allow transition between both states, while maintaining protein structure stability. This composite scheme will henceforward be referred to as ComDYN (COMmon constraints DYNamics). The detailed computational set-up is provided in the Supporting Methods (Feenstra, 2019b).
Essential dynamics analysis was performed on the GLUT1 and GLUT3 simulations using the built-in analysis tools of GROMACS. To allow this comparison between these two homologous proteins, and allow for focusing on overall motions of the transporter region, we selected the structurally conserved helical segments, as summarized in Supporting Table S2, available as extended data (Feenstra, 2019a). Then, the covariance and eigenvalue calculation were performed on the ensemble of both wild-type systems, using the coordinates of the C-alpha atoms for the full atomistic (AT) simulations, and the backbone particle at the C-alpha position for the coarse-grained (CG) and ComDYN simulations.
To analyse the transporter dynamics from the ComDYN, we defined several order parameters as previously proposed by Nagarathinam et al. (2018) by measuring distances between adjacent TM helices, at the intracellular (in)- and extracellular (out)sides of the protein (see Figure 1B, C). For each of the ring of six central helices that make up the solute transporter region, TM2, TM1, TM5, TM8, TM7, TM11 (and back to TM2), we defined an ‘inside’ and ‘outside’ segment of ten residues (See Figure 1 and extended data, Supporting Figure S3 (Feenstra, 2019a)). Comparing the distances of the mutations to both wild types allows us to capture abnormal behaviour and identify the mutations that have the highest impact on the opening and closing mechanism of the apo-form of the transporter protein (see Results and Discussion).
To compare the distributions of sampled distances during the simulations, two metrics were employed: overlap and shift. ‘Overlap’ is the fraction of overlap between both distributions, as the integral of the minimum of both functions. ‘Shift’ quantifies the direction of change, and is obtained by taking the difference in the position of the peak of the two distributions from the ensemble of the simulations (typically, mutation version wild-type). Negative indicates a ‘closing’ motion, positive is ‘opening’. This analysis was performed using the script ‘calc_overlap.py’, which may be found in the extended data.
Table 1 lists the mutants of GLUT1 and GLUT3 that we will consider here, which are situated in the conserved RXGRR-motif distal to the transporter region. The table lists the predicted impact upon mutation obtained from SIFT (Sim et al., 2012) and PolyPhen-2 (Adzhubei et al., 2010). Most mutations are classified as likely pathogenic by both methods, with the exception of GLUT1 R93Q, and GLUT3 R91C and R91H. However, these methods are trained on the dbSNP database which also includes these known mutations, so this should be no surprise. Moreover, these predictions do not allow us to gain any insights into the mechanism by which these mutations may affect transporter function.
The pathogenic mutations in GLUT1 are underlined.
| Protein | Mutation | Clinical significance * | SIFT | PolyPhen |
|---|---|---|---|---|
| GLUT1 | G91D | (Klepper et al., 2001; Klepper & Voit, 2002) GLUT1DS1 omim:606777 | affect protein function | probably damaging |
| R92Q | (Leen et al., 2010) dbSNP rs779073410 significance unknown | affect protein function | probably damaging | |
| R92W | (Schneider et al., 2009) GLUT1DS2 omim:612126 | affect protein function | probably damaging | |
| R93Q | dbSNP rs80359815 significance unknown | tolerated | probably damaging | |
| R93W | (Joshi et al., 2008) GLUT1DS2 omim:612126 | affect protein function | probably damaging | |
| R333W | (Klepper & Voit, 2002; Klepper et al., 2001) EIG12 omim:614847 | affect protein function | probably damaging | |
| R334Q | ClinVar rs892715050 significance unknown | affect protein function | probably damaging | |
| GLUT3 | G89V | dbSNP rs758117298 significance unknown | affect protein function | probably damaging |
| R90W | not reported in dbSNP ( rs1270428275 R90T ) | affect protein function | probably damaging | |
| R91C | dbSNP rs756172777 significance unknown | affect protein function | benign | |
| R91H | dbSNP rs145936296 significance unknown | tolerated | benign | |
| R331K | dbSNP rs770855736 significance unknown | affect protein function | probably damaging | |
| R331S | dbSNP rs749200071 significance unknown | affect protein function | probably damaging | |
| R331W | not in dbSNP significance unknown | affect protein function | probably damaging |
Firstly, we want to verify if our common constraints-based approach for coarse-grained MD simulations (ComDYN) is able to sample the intermediate states between the IO and OO states. Including only elastic network constraints that differed less than 1Å between the IO and OO states in ComDyn, resulted in 1025 constraints (39.9% of the 2568 in the Martini elastic network) for the OO state and 978 (42.2% of 2315) for the IO state. We performed essential dynamics (ED) analysis (Amadei et al., 1993; Van Aalten et al., 1997) to compare AT, traditional CG MARTINI, and ComDYN simulations, as described in the Methods. Figure 2 shows 2D plots of the first two (largest) ED eigenvectors, representing the extracted correlated motions over the ensemble of our simulations. The first eigenvector (EV1) represents the major conformational changes between the OO and IO states, as also indicated by the RMSD values between starting states. The changes observed along EV2 may also be genuinely part of the state transition, however we cannot exclude the possibility that some of these conformational changes may relate to differences between the forcefields used. The sampling of the different states, inward-open and outward-occluded, in AT simulation hardly converges due to their limited time scales. The regular CG simulations reach much longer timescales, and already sample more intermediate conformations, but there is no overlap. The ComDYN simulations, on the other hand, also sample many states intermediate to the inside-open and outside-open starting states, compared to the other simulations. This shows that improved sampling of large conformational transitions may be attained using this approach of CG and ComDyn simulations.
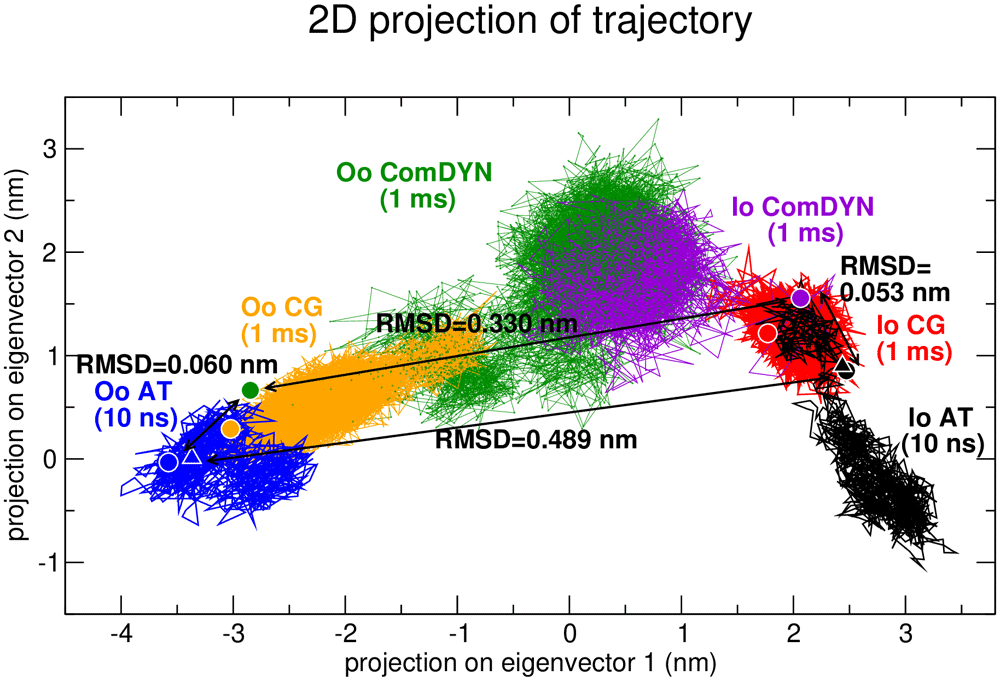
In this projection, eigenvector 1 corresponds to changes from OO (left) to IO (right), showing also the overlap and differences between the AT (full atomistic), the CG (coarse-grained), and the ComDYN (common constraints CG) simulations. Note that there is considerable overlap in the sampling, but that the time-scale of the AT simulations only samples conformations around the IO and OO states and the elastic network in the CG simulations also limits the visited conformations, while the ComDYN samples a large number of conformations between both states.; IO, inward open state (PDB-ID: 5EQI); OO, outward occluded state (PDB-ID: 4ZW9). Spheres indicate the respective starting conformations for each method, triangles to show the corresponding crystal structures; black arrows and labels indicate the RMSD between the starting conformations of AT and ComDYN simulations.
To probe the degree of the conformational changes during ComDyn simulations of the wild types and the mutants in more detail than done with the ED analysis, two distances were used to describe the opening and closing of the periplasmic and cytoplasmic sides of the transporter. Nagarathinam et al. (2018) studied a bacterial homolog of GLUT1 and GLUT3, and analysed the movement between TM5 and TM8. In the extended data, Figure S4 (Feenstra, 2019a), we can see that the distributions obtained from our ComDYN simulations, resemble those reported by Nagarathinam et al. (2018), providing an independent validation that our ComDYN approach is able to sample biologically relevant conformational states for large scale motions, such as those involved in the glucose transporter mechanism. Nevertheless, there are differences between the distance distributions in our work and that of Nagarathinam et al. (2018), which could, apart from obvious differences comparing human glucose transporters with a bacterial multidrug transporter (see also extended data, Figure S4 and Table S3 (Feenstra, 2019a)), also arise from differences in the sampling protocols applied in the two studies.
Therefore, in addition to the TM5-TM8 distances, we extended the analysis to other helices along and across the rim that make up for the entire SLC transporter architecture, allowing us to monitor changes in their position (see Figure 1C, D). For each of these order parameters, we calculated the distance at the inside and outside of the protein with respect to the membrane. Using this analysis, we can immediately observe the changes occurring between the inward-open and outward-occluded states. We see several distances changing significantly during this process: TM1/TM2(in), TM1/TM5(out), TM1/TM7(out), TM1/TM8(in), and TM5/TM11(in) are all closing, while TM1/TM2(out), TM1/TM5(in), TM1/TM7(in), TM2/TM11(in), TM2/TM8(out), and TM5/TM11(out) are opening (see extended data, Table S3 and Figure S4 for more details (Feenstra, 2019a)); these motions are also schematically summarised in extended data, Figure S3 (Feenstra, 2019a). Table 2 summarises the overlap and shift between the sampled distributions of distances between the wild type and each of the mutants for TM5 and TM11 that exhibited the strongest effects and conformational changes during the simulations (the complete table of the distributions for all order parameters are available in the extended data, Table S3 (Feenstra, 2019a)). The corresponding conformational distributions from the ComDYN simulations, calculated as a function of the inner and outer distances between TM5 and TM11 are given in Figure 3.
Figure 3 shows the corresponding distance plots for some of the mutants. These are quantified using the fraction of overlap between both distributions in sampling distributions of sampled conformations, calculated as the integral of the minimum of both functions, i.e., the volume (normalized to a maximum of one), given in %, of the wild type and mutant (small value is large change), and the shift of the peak location (in nm including the direction; positive is to larger distances). The pathogenic mutations are underlined. All the large shifts (absolute above 0.15 nm) and small overlaps (below 0.6 nm) are set to bold. For a visual aid, the shifts are set in italic.
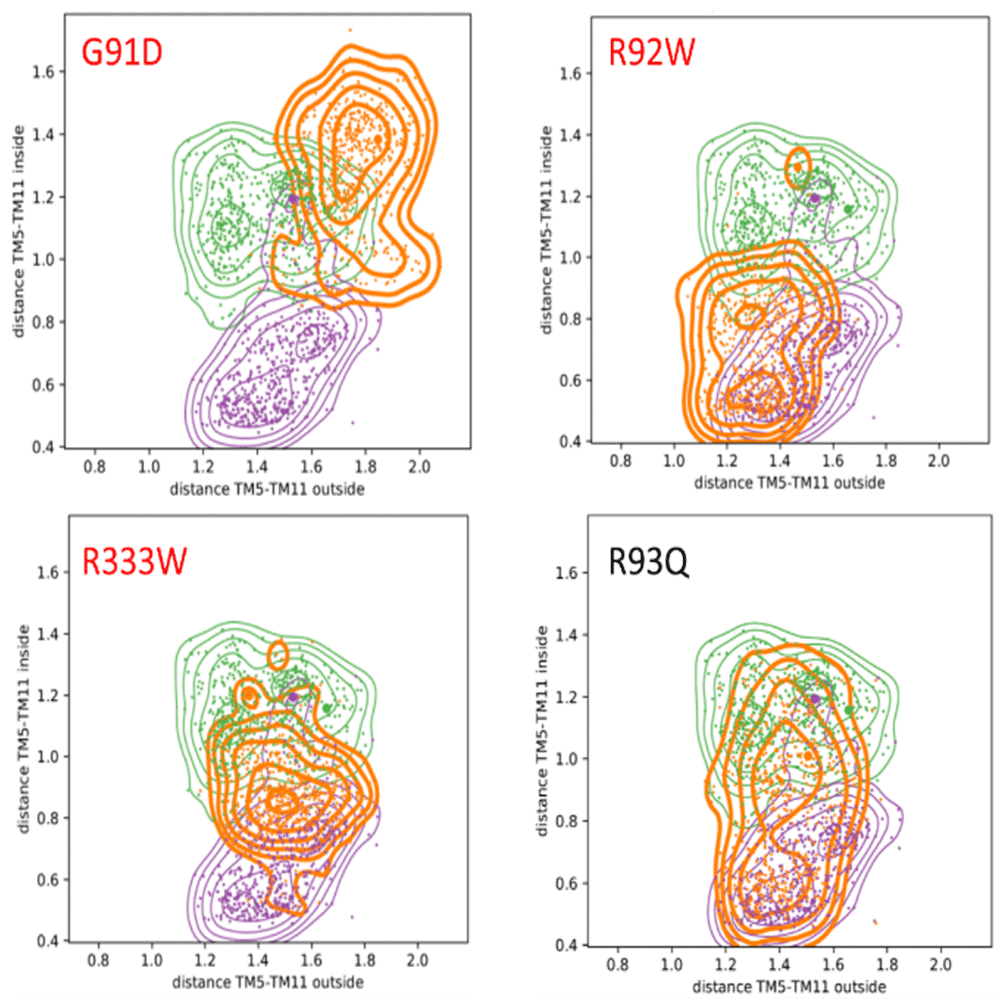
Colour code: wild types IO in purple (PDB-ID: 5EQI), OO in green (PDB-ID: 4ZW9), mutants in orange. Pathogenic mutants are highlighted in red. It should be noted that in contrast to the benign R93Q mutant, the pathogenic mutants do not sample the IO and OO states during the simulation, which strongly indicates that the mutation blocks the proper opening and closing mechanism. Corresponding plots for all mutations are in the extended data, Figure S4. The corresponding quantification of these plots provided as shift and overlap are given in Table 2.
Not all mutations have a high impact on the overall dynamics (extended data, Table S3 and Figure S4 (Feenstra, 2019a)). However, in GLUT1, the reported pathogenic mutation G91D has a profound effect on the dynamics of the protein (i.e., a low overlap and large shift, see Table 2). Also when we consider the distance of TM5-TM11, as shown in Figure 3, the strongest effect is observed for the pathogenic G91D mutant: its distribution varies strongest from the wild types, and hardly visits the inward-open and outward-occluded states. Furthermore, Figure 3 shows that for the pathogenic R92W and R333W mutations, only one state or small parts from both can be accessed. For the benign mutant R93Q, in contrast, it can be seen that both states, inward-open and outward-occluded, are sampled thoroughly during the simulations. Assuming that the relative distance between the two helices is crucial for the correct functioning, this strongly suggests that the pathogenic mutations directly affect the opening and closing mechanism of the GLUT1 transporter.
Mutations in GLUT3 show similar behaviour in TM dynamics compared to those in GLUT1. Here, two mutants with strong abnormal behaviour can be identified: G89V and R91H (Table 2; extended data Figure S4 (Feenstra, 2019a) shows the corresponding distance distribution plots). Additionally, similar to the observations for pathogenic mutations on GLUT1, these mutations no longer sample intermediate states associated with the transport function, unlike the wild type and many of the other mutations. This strongly suggests that the corresponding mutations between GLUT1 and GLUT3 also have the same direct blocking effects on the opening and closing mechanism of the GLUT3 transporter. However, it should be noted that we cannot make any conclusions about the clinical significance of these GLUT3 mutants, as none have been reported to be pathogenic. A next step to elucidate the complete pathway would be to extend the analysis to the outward-open conformation, and include ligand-bound states (Delemotte, 2019), which may shed further light on some of the currently still unexplained pathogenic mutations.
Using extensive ComDYN simulations of GLUT1 and GLUT3 wild type and several clinically relevant mutations, we provide an effective way to study dynamic effects of mutations on the molecular mechanism of human glutamate transporter proteins. Without using full-atomistic details, we were able to get insight into the opening and closing mechanisms, which may account for the (dys)function of the SLC family caused by pathogenic mutations around the conserved RXGRR-motif. Through these mutations (especially G91D, R92W and R333W in GLUT1), we observe that the distances between TM5 and TM11 across the rim of the solute carrier structure are affected the strongest. For this reason, we chose them as our order parameter to explain the abnormal behaviour in the dynamics of the transporter opening and closing mechanism for some of the observed mutants. It should be mentioned that this does not provide an ultimate order parameter to explain all the effects of the pathogenic mutations, but allows us to better understand some of the effects caused by these pathogenic mutations. Comparing atomistic (AT), coarse-grained MARTINI (CG), and ComDYN simulations, our work shows that our CG ComDyn simulations are sufficiently accurate to sample the intermediate states between the conformational states and capture some of the effect of the mutations on the dynamic and function of these transporter proteins. This helps to elucidate the effects of pathogenic mutations on the structure and dynamics of the GLUT1 and GLUT3 transporters which were previously not understood.
Crystal structures for GLUT1 IO state (Deng et al., 2015) and for GLUT3 OO state (Kapoor et al., 2016) were obtained from the Protein Data Bank, under accession numbers 5EQI and4ZW9, respectively.
Open Science Framework: ComDYN. https://doi.org/10.17605/OSF.IO/F82H5 (Feenstra, 2019a).
The following extended data are available:
Data.tgz. data files accompanying analyses performed in this study.
Table S1. Summary of the molecular composition of simulated systems.
Table S2. Structurally conserved helical segments between 4ZW9 and 5EQI.
Table S3. Wild-type and Mutant simulations compared by Overlap and shift between TM helix distance distributions.
Figure S1. Sequence alignment between E. coli multi-drug transporter MDFA, and human glucose transporters GLUT1 and GLUT3.
Figure S2. Pipe representation of the inward-open conformation of the transporter.
Figure S3. Schematic view of the observed pore mechanism going from the inward-open state to the outward-occluded state.
Figure S4. Distribution of inside and outside helix distances for all examined mutants in GLUT1 and GLUT3.
Extended data are available under the terms of the Creative Commons Zero "No rights reserved" data waiver (CC0 1.0 Public domain dedication).
Scripts used to setup and analyze the ComDYN simulations available from:
https://github.com/ibivu/ComDYN.
Archived source code at time of publication: https://doi.org/10.5281/zenodo.2591477 (Feenstra, 2019b).
License: GNU General Public License 3.0.
This research (in part) used computational resources of COMA and HA-PACS provided by Multidisciplinary Cooperative Research Program in Center for Computational Sciences, University of Tsukuba, Japan.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Clinical genetics; Inborn errors of metabolism; GLUT1 deficiency syndrome
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Membrane proteins, ion channels, molecular dynamics simulations, enhanced sampling
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Partly
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular simulation of proteins and DNA
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
No
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Membrane proteins, ion channels, molecular dynamics simulations, enhanced sampling
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | |||
|---|---|---|---|
| 1 | 2 | 3 | |
|
Version 2 (revision) 13 Jun 22 |
read | read | |
|
Version 1 22 Mar 19 |
read | read | |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)