Keywords
Plasmodium falciparum, Plasmodium vivax, malaria, animals, host reservoir, PCR.
In Indonesia, malaria incidence is at a high rate despite maximum preventive efforts. Therefore, this study aims to determine the possibility of a Plasmodium reservoir among domestic animals in malaria-endemic areas.
Animal blood was collected using EDTA tubes, then smeared and stained with Giemsa for Plasmodium microscopic identification. About 10 μl of blood was dropped on to a filter paper to capture Plasmodium DNA. Nested PCR was used for parasite molecular detection, while Plasmodium species were identified using the sequenced DNA.
A total of 208 and 62 animal blood samples were collected from Gaura village, West Sumba and Fakfak village, West Papua, Indonesia respectively. In total, 32 samples from Gaura contained P. falciparum or P. vivax, while the Plasmodium percentage in buffalo, horse, goat, and dogs were 20.7%, 14.3%, 5.8%, 16.7%, respectively. P. knowlesi was not found in any of the samples, and no other species were detected in 18 pig blood samples.
The human Plasmodium DNA in domestic animals within malaria-endemic regions suggests a potential link to the persistence and high prevalence of malaria in these areas. While the findings suggest a potential role of domestic animals in malaria transmission, they remain preliminary and do not definitively establish domestic animals as reservoirs. Further research is necessary to confirm these findings and to better understand the contribution of domestic animals to the transmission dynamics of malaria.
Plasmodium falciparum, Plasmodium vivax, malaria, animals, host reservoir, PCR.
This revised version of the manuscript introduces several important updates that strengthen both the theoretical framework and methodological clarity. A more discussion-detailed exploration of the coevolution. We have also addressed the ecological and evolutionary barriers that make the hypothesis of host-switching to domestic animals less plausible, adding empirical and theoretical evidence to support the biological feasibility of such a transition.
We have included more detailed information regarding primer design, validation, and potential cross-reactivity concerns, ensuring the methodology is transparent and reproducible. These revisions enhance the manuscript's robustness, improving its scientific rigour and addressing concerns raised in the peer review process.
See the authors' detailed response to the review by Jaishree Raman
See the authors' detailed response to the review by Paul Cliff Simon Divis
See the authors' detailed response to the review by Alfred Amambua-Ngwa
Indonesia is striving toward becoming a malaria-free country through the Malaria Control Programme. Despite the implementation of several efforts, such as early detection, treatment, and mosquito vector eradication, the disease remains a persistent challenge. Factors including parasite resistance to antimalarial drugs, mosquito resistance to insecticides, inadequate health system performance, and host reservoir presence contribute to continuous malaria prevalence.
Plasmodium falciparum and P. vivax commonly infect humans, leading to high morbidity and mortality. Only humans were considered the primary host for Plasmodium species including P. falciparum, P. vivax, P. malariae, and P. ovale before molecular diagnostics development. However, studies over the past two decades reported that the parasites originated from animals. Specifically, P. falciparum was traced back to gorillas (Liu et al., 2010) and chimpanzees (Duval et al., 2010; Krief et al., 2010), while P. vivax was associated with African apes (Liu et al., 2014). Other sources stated were the origination of P. malariae from chimpanzees (Duval et al., 2010) and P. knowlesi from monkeys (Knowlesi, 1935; Zhang et al., 2016), while P. ovale found in humans and chimpanzees had genetic similarities (Duval et al., 2009). These findings underscore the potential for zoonotic transmission of Plasmodium species from non-human primates to humans, a process that is likely facilitated by ecological changes such as habitat loss and human encroachment into forested (Davidson et al., 2019). Forest workers, due to their proximity to wildlife, face an elevated risk of malaria transmission, as demonstrated by studies conducted in South Kalimantan (Rahayu et al., 2016). This information suggests that the proximity between domestic animals and humans may also play a role in cross-species transmission. This is particularly pertinent given the population of domestic animals living in close proximity to human settlements, which could act as additional reservoirs for Plasmodium parasites affecting humans.
Therefore, the presence of domestic animals in malaria-endemic areas has become the focus of our study to explore their role in exacerbating the local epidemiological situation. Specifically, the high incidence of malaria in Gaura village in West Sumba and Fakfak in West Papua provides compelling evidence for the need for further investigation into the potential role of domestic animals in malaria transmission. The Annual Parasite Incidence (API) in Fakfak, West Papua, and East Nusa Tenggara, West Sumba, were reported at 4.85% and 12.9%, respectively (Public Health Office of Fakfak and West Sumba, unpublished data).
This study was conducted in October 2018 in Gaura village, West Sumba Regency, an area 29.96 km2 in size inhabited by 9,584 people, and Fakfak, West Papua Province, in August 2019 with an area of 11,036 km2 inhabited by 84,692 people ( Figure 1). The residents’ main occupation is farming, while livestock such as goats, horses, cows, pigs, and buffalos are commonly found in their enclosures located around the owner’s residence. Furthermore, they also own pets such as dogs and cats. The average distance between enclosure and home in Fakfak was 225 meters while in Gaura village the distance was 0-10 meters.
Sampling was carried out by the veterinarian and staff from West Sumba and Fakfak Animal Husbandry Office. The buffaloes, goats, pigs, and horses’ blood samples were collected in 5 ml EDTA tubes from the jugular vein located in the ventrolateral area of the neck using vacutainer needles, size 16–18. Meanwhile, the dog’s blood was drawn from the cephalic antebrachial vein in the leg using a size 21 vacutainer needle. By using a sterile micropipette, approximately 10 ul of EDTA blood was dropped onto a microscope slide, then smeared and stained with Giemsa (MERCK Millipore, Germany) for Plasmodium microscopic identification, while the remaining blood was dropped by sterile micropipet (about 20 ml) onto a filter paper (Whatman CAT No. 1442-090) until it absorbed to about 1.5 cm in diameter and hold back until blood at filter paper dry. The dry filter paper was put on a sterile clip seal plastic bags and stored at room temperature for a maximum of 10 days. All the sample collection process was done by sterile conditions.
A dried blood spot (DBS) isolation kit for DNA extraction on filter paper (Cat. no. 36000) from Norgen Biotec was used. A 6 × 3 mm piece of blood-stained filter paper was put into a 1.5 ml tube containing 100 μl of digestion buffer B. It was vortexed and incubated at 85°C for 10 minutes. Afterwards, 20 μl of proteinase K and 300 μl of lysis buffer B were added to the tube and then vortexed before incubation at 56°C for 10 minutes. About 250 μl of 95% ethanol was added to the tube and then vortexed, while the DNA content was washed by adding 500 μl of WN wash solution and centrifugated for one minute at 8,000 rpm. Washing was carried out again using 500 μl of WN wash solution and centrifugated at 14,000 rpm. For DNA elution, 90 μl of elution buffer B was put into the tube and centrifuged at 8,000 rpm for one minute, and the purified DNA was stored at -20°C.
DNA amplification by nested PCR and qPCR were performed as directed by Tiangen Biotech (Beijing). Plasmodium DNA amplification was carried out using the nested PCR method with a 2× Tag Plus PCR mix enzyme (Tiangen). The final volume of 12.5 μl contained 6.25 μl enzyme, 2.25 μl ddH2O, 1 μl forward primers, 1 μl reverse primers, and 2 μl DNA sample. For sequencing, the PCR mixture’s volume was doubled, with the final volume being 25 μl, while the primer sequences of P. falciparum, P. vivax (Snounou et al., 1993a) and P. knowlesi (Lee et al., 2011) can be seen in Table 1.
The nested one DNA amplification temperature was set at 94°C denaturation (one minute), 55°C annealing (one minute) and 72°C extension (one minute) for 35 cycles. For nested two, denaturation was carried out at 94°C (30 seconds), 55°C annealing (one minute) and extension was at 72°C (30 seconds) in 35 cycles. There was a difference in the annealing temperature for each species in nested two, namely 55°C (one minute) for PCR multiplex P. falciparum and P. vivax, but 56°C (one minute) for P. knowlesi. Nested one products were used as templates for nested two and both were run on agarose gel 1.5% and 2%, respectively, while qPCR was run on agarose gel 1.5% and view in a gel documentation system. All the stage of DNA amplification was carried out in sterile media and places such us laminar airflow. Molecular work was not performed for P. ovale and P. malariae due to difficulties in finding the positive control, and according to the local health office these species have never been reported from Sumba and Fakfak.
Preventing the possibility of positive contamination of DNA Plasmodium in the first extraction, DNA was re-extracted from the same blood spot at filter paper. The extraction room used was confirmed to have never been used for Plasmodium extraction sample previously. Filter papers were cut by scissors which has been sterilization. PCR was performed using the primers, rPF1 and rPF2, as well as rPV1 and rPV2 (Snounou et al., 1993b) to detect P. falciparum and P. vivax, respectively. The same extraction and amplification method were used as described above.
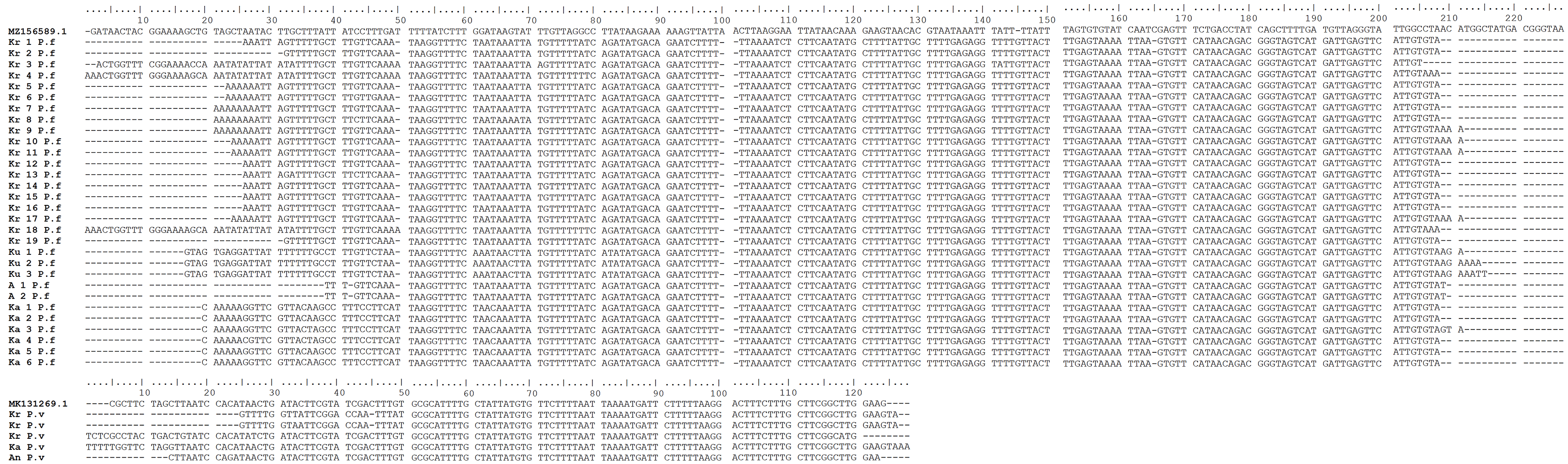
To determine the Plasmodium species, in the second round of nested PCR, products having positive band targets were sent to the 1st BASE, Axil Scientific Pte Ltd Singapore for sequencing. The DNA sequence result was adjusted using multiple alignments found in the BioEdit 7.0 application (Hall et al., 2011) and then read by the BLAST program from the NCBI website.
This study was approved for ethical clearance by the ethics committee of the Faculty of Medicine, Hasanuddin University (734/H4.8.4.5.31/PP36-KOMETIK/2018). All efforts were made to ameliorate any suffering of animals. To prevent stress, animals were comforted by their owners while blood samples were taken, and sampling was performed by experienced officers. Second and third blood samples were taken if there was a failure in the first sample and only if the animals were cooperative. About 20% of animals were sampled more than once.
A total of 208 and 62 blood samples were collected in Gaura and Fakfak villages, respectively, from 92 buffalos, 21 horses, 121 goats, 18 dogs, and 18 pigs. Tests conducted using the nested PCR method identified 32 of the 270 animals as positive for P. falciparum and P. vivax. Furthermore, 20.7% buffalo, 14.3% horse, 5.8% goat, and 16.7% dog were Plasmodium-positive, with one buffalo showing mixed infections of P. falciparum and P. vivax. P. knowlesi was absent in all the blood samples and no form of malaria parasites was found in the 18 pigs. Additionally, PCR gel products, DNA sequence results, and the quality of samples can be seen in Figures 2, 3, and 4, respectively (Munirah et al., 2021). Plasmodium distribution in samples from Gaura and Fakfak are presented in Table 2, showing that blood containing the malaria parasites was only found in Gaura. The results of qPCR performed using rPF1–rPF2 and rPV1–rPV2 primers were similar to those of the nested PCR.
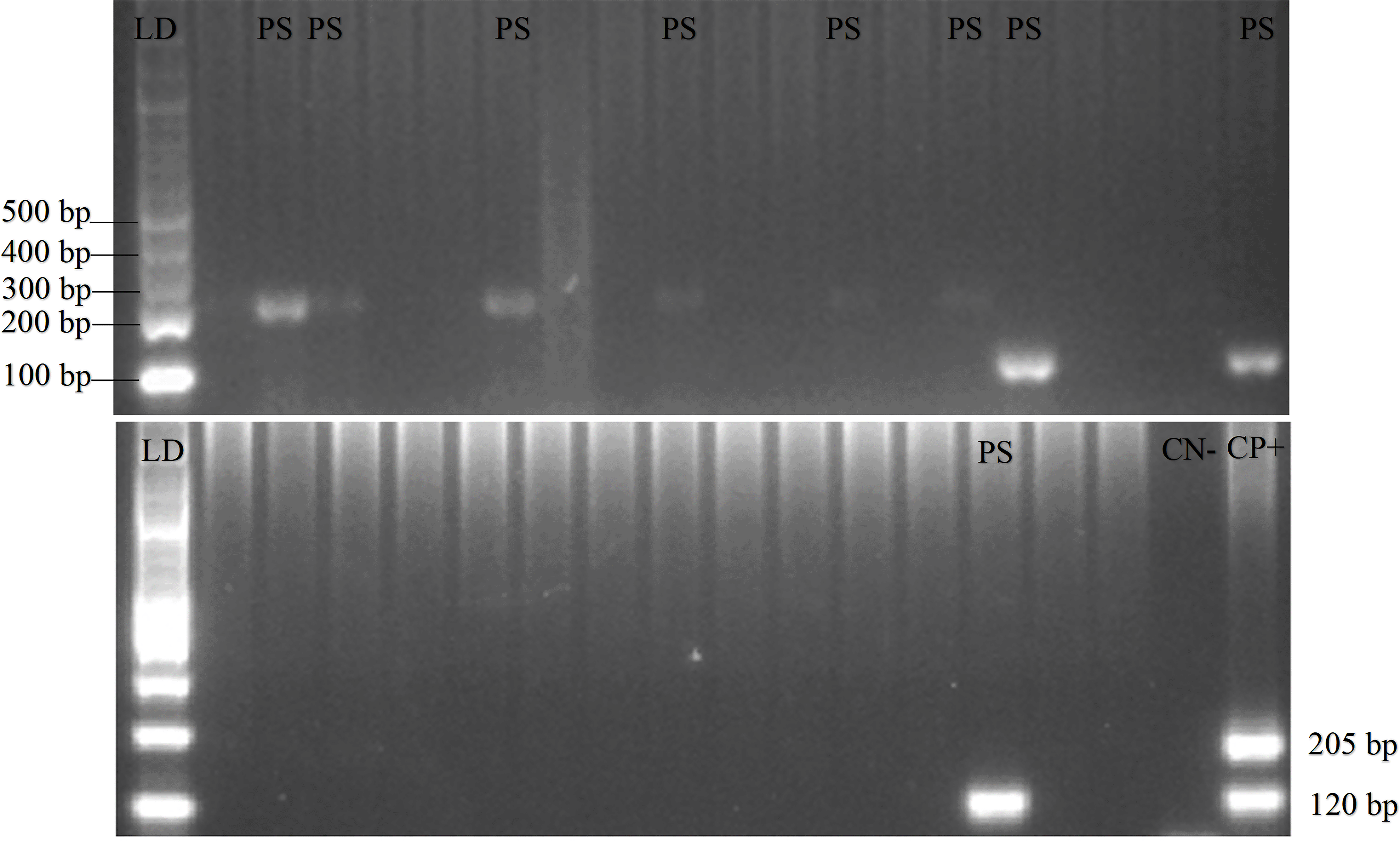
120 bp for positive Plasmodium vivax, 205 bp for positive Plasmodium falciparum.
Microscopy identified Plasmodium-like structures in some samples at 100× magnification can be seen in Figure 5. P. falciparum gametocytes-like found in buffaloes were sausage and crescent-shaped (a, b), while schizonts found in horses were smaller or the same size as the red blood cells (c). The P. vivax gametocyte-like was larger than the red blood cells found in buffalo (d). P. falciparum gametocyte-like and trophozoites (ring-shaped) with one or two nuclei was found in goats (e) and P. falciparum trophozoite-like found in horses had one nucleus (f).
Plasmodium presence was suspected in domestic animals because malaria cases in both Gaura and Fakfak villages remained high despite applying maximum preventive efforts including insecticide-treated bed nets. The results showed that 32 of the 270 blood (11.9%) samples contained human Plasmodium, serving as the first data report regarding this parasite, hence further investigation should be conducted.
The specificity of the nested PCR was ensured by using primers designed from conserved regions unique to Plasmodium species, as described in previous studies (Snounou et al., 1993b). All assays included negative controls to rule out contamination. Positive PCR products were sequenced, and BLAST analysis confirmed that the amplified fragments matched the target Plasmodium species (P. falciparum and P. vivax). In addition to the nested PCR protocol, we used longer primers—rPF1-rPF2 for P. falciparum and rPV1-rPV2 for P. vivax to amplify DNA fragments of 918 bp and 714 bp, respectively. The use of these primers targeting longer DNA sequences provides an additional layer of specificity, as longer amplicons reduce the likelihood of non-specific binding or amplification. The results from these longer primers have been published and can be accessed for detailed evaluation at https://doi.org/10.6084/m9.figshare.14703012.v3. This additional evidence underscores the robustness and validity of our findings. Furthermore, we performed re-extraction and re-amplification of DNA from the same samples, which yielded consistent results, strengthening the conclusion that the nested PCR results are neither due to cross-reactivity nor contamination.
Previous studies found P. relictum in avian species (Cox, 2010), P. cephalophi in ungulates (Bruce et al., 1913), P. traguli in mousedeer (Garnham and Edeson, 1962), P. brucei in gray duiker (Bruce et al., 1915; Templeton et al., 2016a), P. bubalis in water buffalo (Sheather, 1919), and P. odocoilei in white-tailed deer (Garnham and Kuttler, 1980; Perkins and Schaer, 2016). Other parasites detected included P. caprae in goats (ruminant) (Kaewthamasorn et al., 2018), P. bergei in Rodentia (Vincke and Lips, 1948), as well as P. cynomolgi, P. inui, and P. fragile in primates (Dixit et al., 2018). The five Plasmodium species infecting humans were originally parasites in primates (Duval et al., 2010; Knowlesi, 1935; Liu et al., 2014; Ng et al., 2008; Singh and Daneshvar, 2013; Zhang et al., 2016). This study identified P. falciparum in buffalos, goats, dogs, and horses, as well as P. vivax in buffalos, goats, and dogs. Initially, Plasmodium presence in the erythrocytes (RBCs) of these animals was uncertain, but the nested PCR produced the same results for all positive samples. Sequencing analysis of positive bands in the nested PCR confirmed the presence of P. falciparum and P. vivax ( Figure 3). The number of positive Plasmodium cases in buffalos was higher because of susceptibility to parasitic infections. Another source declared age as one of the factors predisposing buffalos to high risk of infections (Nurhidayah et al., 2019), with the average age exceeding seven years. This study provides the first report of human Plasmodium in ruminant, ungulate, and carnivorous domestic animals.
Investigations conducted by Templeton in Thailand in 2008 and 2015 showed the presence of P. bubalis among buffalos. The microscopic appearance of P. bubalis was depicted in the journal published by Templeton et al. (2016b). However, this current study did not detect any similarity between P. bubalis and P. falciparum gametocytes in buffalos. A molecular examination method targeting the cytochrome b (cytb) gene identified the presence of P. caprae in goats from Thailand, Myanmar, Iran, Kenya, and Sudan. The trophozoites of P. caprae can be observed in a journal published by Kaewthamasorn et al. (2018).
The discovery of Plasmodium among domestic animals in malaria-endemic areas raises the following questions. How do P. falciparum and P. vivax survive in these animals? Can animals serve as intermediate hosts for these parasites? Have both species evolved to live in ruminants, ungulates, and carnivores? Due to repeated exposure, have these animals become more susceptible to Plasmodium, which generally infects humans? Is Plasmodium pathogenic in animals?
P. knowlesi is identified as a commensal microbe in primates but pathogenic in humans (Jongwutiwes et al., 2004; Ng et al., 2008; Singh and Daneshvar, 2013), and the transmission to humans can be attributed to forest loss or the invasion of primate habitats (Davidson et al., 2019). There is a possibility that the proximity of animals and humans facilitates easier transfer of parasites between both groups through mosquitoes. In humans, P. falciparum and P. vivax infect by initially growing in liver cells before moving to RBCs. The parasites multiply in RBCs, leading to medical conditions characterized by fever, chills, headache, profuse sweating, weakness, rheumatic pain, symptoms of anemia or lack of blood, and nausea or vomiting. Plasmodium found in non-primates remains undetermined as pathogenic or commensal. However, sporozoites of P. brasilianum identified in animals migrate directly to the liver where multiplication occurs, releasing merozoites. The merozoites infect RBCs, initiating symptomatic disease in these animals (Erkenswick et al., 2017). Organisms infected by Plasmodium become symptomatic when the parasite cycle advances to the erythrocyte stage or causes malaria due to the rupture of RBCs. The identification of intermediate hosts for P. falciparum and P. vivax in livestock is still a challenge. Furthermore, various studies attempted to provide evidence for the origination of these two species from chimpanzees and gorillas.
The investigation conducted by Prugnolle in 2013 signified that P. vivax detected in monkeys and humans had similarities. The results showed the possibility of natural transfer between the two organisms, particularly in environments where animals and humans coexist, facilitating continuous parasite transmission through vectors (Prugnolle et al., 2013). Similarly, a study by Mu in 2005 described P. vivax as a zoonotic parasite (Mu et al., 2005).
High API was observed in Fakfak and Gaura villages, but only animals from Gaura had human Plasmodium. This difference may be attributed to the long distance of approximately 50–500 m between the residential houses and animal enclosures in Fakfak. Meanwhile, in Gaura, residents live in stilt houses, with the ground floor and surroundings serving as a shelter for animals, which facilitate microbial transfer between humans and animals through mosquitoes. The inaccessibility of sampling locations and steep geographical conditions in Fakfak posed challenges during sample collections. Moreover, the vector census conducted in both areas identified 11 Anopheles mosquito species in Gaura village, including An. vagus, An. sundaicus, An. aconitus, An. Kochi, An. flavirostris, An. indefinitus, An. maculatus, An. minimum, An. annularis, An. nivipes, and An. subpictus. Molecular examination results showed that two An. sundaicus were infected by Plasmodium, while no Anopheles was found in Fakfak. The species An. sundaicus was identified as zoophilic in India (Vidhya et al., 2019) and anthropophilic in Mekong, Vietnam (Trung et al., 2005). These different reports from both areas suggested variations in the behavior of An. sundaicus, showing the diversity of mosquito biting patterns influenced by environmental factors. The proximity between domestic animals and humans in Gaura village may increase the tendency of bites from An. sundaicus, thereby necessitating further investigations.
The application of livestock for zooprophylaxis in malaria-endemic areas offers several advantages but tends to increase the survival of mosquitoes, which become potential disease vectors. Proximity between infected and uninfected organisms is a significant factor driving parasite transmission (Kuris et al., 1980). The study by Hasyim suggests that livestock in endemic areas have a high potential to increase malaria incidence in Indonesia (Hasyim et al., 2018). Moreover, zoopotentiation often occurs when livestock are kept indoors or near houses (Donnelly et al., 2015). Locating livestock away from humans tends to reduce malaria cases (Franco et al., 2014). Strategies for separating livestock from humans to minimize mosquito bites in both groups are essential. Mosquitoes that have fed on the blood of animals tend to not suck blood from humans. However, closeness between animals and humans can lead to massive pathogen transmission through intermediary vectors.
Vector behavior, particularly in terms of blood-feeding, plays a crucial role in accelerating host switching by facilitating the transfer of pathogens between different host species. Vectors, can act as bridges between animal and human, allowing pathogens to move from one species to another. Vector behavior, including host preference, feeding frequency, and survival capacity in various environments, is heavily influenced by ecological and physiological factors inherent to the vector itself (Ellwanger and Chies, 2021). The impact of vector behavior on host switching can be better understood through behavioral ecology studies that consider the interactions between vectors, hosts, and pathogens within their natural environmental context. An understanding of the vector’s blood-feeding patterns, including activity timing and host preferences, is essential in designing disease control strategies (Tisgratog et al., 2012). For example, if vectors are more likely to feed on infected domestic animals, this behavioral pattern can increase the likelihood of pathogen transmission to humans. Thus, changes in vector behavior related to adaptation to the human environment can accelerate host switching processes and facilitate the emergence of new zoonotic diseases (Obeagu and Obeagu, 2024).
One of the primary evolutionary pressures driving host switching is selection for adaptation, which enables pathogens or parasites to optimize their ability to exploit new hosts. This adaptation may involve changes in host recognition mechanisms, strategies to evade the host’s immune system, and metabolic pathways that allow the pathogen to utilize resources from the new host. For example, a pathogen that has evolved in a specific host may need to alter its molecular recognition mechanisms or metabolic pathways in order to survive and reproduce in the distinct biochemical environment of a new host (Barber and Fitzgerald, 2024). Although these adaptations enable pathogen transition to a new host, host switching is often constrained by significant physiological barriers. Host-specific factors, such as differences in immune responses, environmental conditions, and biochemical microecosystems, can pose substantial challenges to pathogen colonization in a new host (Bäumler and Fang, 2013). The immune system of a host, having evolved to recognize and combat specific pathogens, can act as a barrier for pathogens attempting to infect a new host with a different immune response (Shivahare, Dubey and McGwire, 2023). Additionally, physiological mismatches between the pathogen and the new host, such as differences in body temperature, pH, or nutrient availability, can hinder pathogen infection or replication in the new host (Prior et al., 2020). The ability of a pathogen to evade the immune system, through mechanisms like antigenic variation or suppression of immune responses, plays a critical role in overcoming these barriers. Furthermore, the evolution of host resistance and pathogen virulence in response to these physiological mismatches is a key factor influencing the success or failure of host switching.
The process of host switching also involves elements of co-evolution between the pathogen and host, creating an adaptive dynamic that unfolds over several generations. Therefore, host switching is not merely a matter of chance or opportunity but is instead shaped by a series of evolutionary interactions between the pathogen and the host’s defense system (Morgan and Koskella, 2011; Yang, Ma and Zu, 2022). Research on host switching is particularly relevant in the context of zoonosis, diseases that can be transmitted between animals and humans, as it provides insights into the factors that either facilitate or hinder the transition of pathogens between host species. For instance, the discovery of Plasmodium in domestic animals in the village of Gaura, which is not found in Fakfak, suggests that proximity between humans and domestic animals may play an important role in facilitating pathogen adaptation and host switching.
Plasmodium can be detected microscopically due to the smaller size of RBCs in animals compared to humans, but the molecular method is more significant for identifying the presence of this parasite. Nested PCR which offered high sensitivity similar to Real-Time PCR at a low cost was applied for the detection process (Green and Sambrook, 2019; Perandin et al., 2004). The microscopic method using double fluorescent dyes with Giemsa stain is recommended for subsequent studies (Guy et al., 2007).
Further investigation is needed to confirm the presence of domestic animal Plasmodium by amplifying the cytochrome B sequence and sequencing the invasion ligands such as DBL protein. Additionally, other challenges in malaria elimination shown by the results of this study should be addressed.
Molecular evidence suggests the presence of human Plasmodium DNA in domestic animals within Gaura village; however, these findings are preliminary and do not definitively confirm animals as reservoirs of malaria. To verify the identity of the parasite and assess its potential role in human malaria transmission, a more extensive genetic analysis is required in future studies. Moreover, additional research on parasite ecology, host specificity, and transmission dynamics is essential to substantiate these results and inform the development of effective malaria control strategies.
Figshare: Underlying data for ‘The discovery of human Plasmodium among domestic animals in West Sumba and Fakfak, Indonesia’, https://doi.org/10.6084/m9.figshare.14703012.v3 (Munirah et al., 2021).
This project contains the following underlying data:
Figshare: ARRIVE checklist for ‘The discovery of human Plasmodium among domestic animals in West Sumba and Fakfak, Indonesia’, https://doi.org/10.6084/m9.figshare.14703012.v3 (Munirah et al., 2021).
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
The author acknowledges the assistance of the West Sumba District and Fakfak Animal Husbandry Service, who helped carry out animal blood collection. We thank the people of Gaura village and Fakfak, who allowed us to take blood samples from their animals. We also thank Syahruni and Handayani Halik from Hasanuddin University Medical Research Center (HUM-RC) for their help with molecular work.
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Is the work clearly and accurately presented and does it cite the current literature?
Yes
Is the study design appropriate and is the work technically sound?
Yes
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Yes
Are all the source data underlying the results available to ensure full reproducibility?
Yes
Are the conclusions drawn adequately supported by the results?
Yes
References
1. Faculty of Medicine, Universitas Diponegoro Semarang, Indonesia, Sumanto D: Human-Plasmodium Like in Domestic-goat Blood in Malaria Endemic Areas in Purworejo Indonesia. Journal of Communicable Diseases. 2021; 53 (04): 148-152 Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Epidemiology, Parasitology, Malaria disease, Helminthiasis
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: molecular epidemiology, population genetics, genomics, zoonotic malaria
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Antimalarial drug and diagnostic resistance, molecular biology, molecular epidemiology, malaria elimination and control
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Antimalarial drug and diagnostic resistance, molecular biology, molecular epidemiology, malaria elimination and control
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Yes
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
Partly
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Antimalarial drug and diagnostic resistance, molecular biology, molecular epidemiology, malaria elimination and control
Is the work clearly and accurately presented and does it cite the current literature?
Partly
Is the study design appropriate and is the work technically sound?
Partly
Are sufficient details of methods and analysis provided to allow replication by others?
Partly
If applicable, is the statistical analysis and its interpretation appropriate?
Not applicable
Are all the source data underlying the results available to ensure full reproducibility?
No
Are the conclusions drawn adequately supported by the results?
Partly
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Malaria, genomics, genetics, immunology, epidemiology, invasion, immunology
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||||
|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |
|
Version 4 (revision) 10 Dec 24 |
read | |||
|
Version 3 (revision) 09 Apr 24 |
read | read | ||
|
Version 2 (revision) 15 Feb 23 |
read | |||
|
Version 1 23 Jul 21 |
read | read | ||
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)