Keywords
Uniprot ID P35637, FUS, RNA-binding protein FUS, antibody characterization, antibody validation, Western Blot, immunoprecipitation, immunofluorescence
This article is included in the YCharOS (Antibody Characterization through Open Science) gateway.
This article is included in the Cell & Molecular Biology gateway.
RNA-binding protein Fused-in Sarcoma (FUS) plays an essential role in various cellular processes. Mutations in the C-terminal domain region, where the nuclear localization signal (NLS) is located, causes the redistribution of FUS from the nucleus to the cytoplasm. In neurons, neurotoxic aggregates are formed as a result, contributing to neurogenerative diseases. Well-characterized anti-FUS antibodies would enable the reproducibility of FUS research, thereby benefiting the scientific community.
In this study, we characterized ten FUS commercial antibodies for Western Blot, immunoprecipitation, and immunofluorescence using a standardized experimental protocol based on comparing read-outs in knockout cell lines and isogenic parental controls.
We identified many high-performing antibodies and encourage readers to use this report as a guide to select the most appropriate antibody for their specific needs.
Uniprot ID P35637, FUS, RNA-binding protein FUS, antibody characterization, antibody validation, Western Blot, immunoprecipitation, immunofluorescence
1. Author list re-ordering and 3 new authors added.
2. Flow cytometry data + Figure 4:
This updated version includes new data in which antibodies were tested by Flow cytometry. As such, methods and figure legends were added. A PNG file for figure 4 was uploaded as well.
3. Table 3:
To guide viewers in assessing the results, we included a table demonstrating the various scenarios that can occur. The PNG files are within the cells of the table.
4. New references + change in order
Please see additional references 11-15, which changed the order of the proceeding references.
See the authors' detailed response to the review by Ryota Hikiami
The fused-in sarcoma (FUS) protein is an RNA-binding protein involved in numerous cellular processes including transcriptional regulation, RNA splicing, RNA transport and DNA repair.1 Predominantly localized in the nucleus, FUS can shuttle between the nucleus and cytoplasm.2 The FUS transcript is reported to have multiple domains including an N-terminal Gln-Gly-Ser-Tyr -rich region, an RNA-recognition motif, Arg-Gly-Gly repeat regions, a zinc finger motif and a highly conserved C-terminal NLS.3–5
Variants in the FUS gene have been identified as potential causative factors for amyotrophic lateral sclerosis (ALS), frontotemporal dementia (FTD) and frontotemporal lobar degeneration.6–9 FUS related mutations found in familial ALS/FTD patients are clustered in the C-terminal NLS, causing FUS to be mislocalized and accumulate as aggregates in the cytoplasm of neurons, initiating a pathway that contributes to neurodegeneration.6,7 FUS function is reduced when aggregates form, but it is not yet known whether this initiates the pathogenic process or if the aggregates are pathogenic.10 Mechanistic studies would be greatly facilitated with the availability of high-quality antibodies.
This research is part of a broader collaborative initiative in which academics, funders and commercial antibody manufacturers are working together to address antibody reproducibility issues by characterizing commercial antibodies for human proteins using standardized protocols, and openly sharing the data.11–13 Here, we compared the performance of a range of commercially-available antibodies for FUS and validated several antibodies for western blot, immunoprecipitation, immunofluorescence and flow cytometry, enabling biochemical and cellular assessment of FUS properties and function. The platform for antibody characterization used to carry out this study was endorsed by a committee of industry academic representatives. It consists of identifying human cell lines with adequate target protein expression and the development/contribution of equivalent knockout (KO) cell lines, followed by antibody characterization procedures using most commercially available antibodies against the corresponding protein. The standardized consensus antibody characterization protocols are openly available on Protocol Exchange, a preprint server (DOI: 10.21203/rs.3.pex-2607/v1).14
The authors do not engage in result analysis or offer explicit antibody recommendations. Our primary aim is to deliver top-tier data to the scientific community, grounded in Open Science principles. This empowers experts to interpret the characterization data independently, enabling them to make informed choices regarding the most suitable antibodies for their specific experimental needs. Guidelines on how to interpret antibody characterization data found in this study are featured on the YCharOS gateway.15
Our standard protocol involves comparing readouts from wild-type (WT) and KO cells.16–20 To identify a cell line that expresses adequate levels of FUS protein to provide sufficient signal to noise, we examined public proteomics databases, namely PaxDB21 and DepMap.22 HeLa was identified as a suitable cell line and thus HeLa was modified with CRISPR/Cas9 to knockout the corresponding FUS gene (Table 1).
| Institution | Catalog number | RRID (Cellosaurus) | Cell line | Genotype |
|---|---|---|---|---|
| ATCC | CCL-2 | CVCL_0030 | HeLa | WT |
| Montreal Neurological Institute | - | CVCL_A8VH | HeLa | FUS KO |
For western blot experiments, we resolved proteins from WT and FUS KO cell extracts and probed them side-by-side with all antibodies in parallel17–20 (Figure 1).

Lysates of HeLa (WT and FUS KO) were prepared and 30 μg of protein were processed for western blot with the indicated FUS antibodies. The Ponceau stained transfers of each blot are presented to show equal loading of WT and KO lysates and protein transfer efficiency from the acrylamide gels to the nitrocellulose membrane. Antibody dilutions were chosen according to the recommendations of the antibody supplier. An exception was given for antibody GTX101810, which was titrated to 1/3000, as the signal was too weak when following the supplier’s recommendation. Antibody dilution used: NBP2-52874* at 1/1000; GTX101810 at 1/3000; GTX01039* at 1/1000; 60160-1-Ig* at 1/10000; 11570-1-AP at 1/4000; MA3-089* at 1/2000; MA5-32483** at 1/1000, ab124923** at 1/5000; ab154141* at 1/1000; ab243880** at 1/1000. Predicted band size: 53 kDa. Observed specific band size: ~70 kDa. *Monoclonal antibody; **Recombinant antibody.
For immunoprecipitation experiments, we used the antibodies to immunopurify FUS from HeLa cell extracts. The performance of each antibody was evaluated by detecting the FUS protein in extracts, in the immunodepleted extracts and in the immunoprecipitates17–20 (Figure 2).
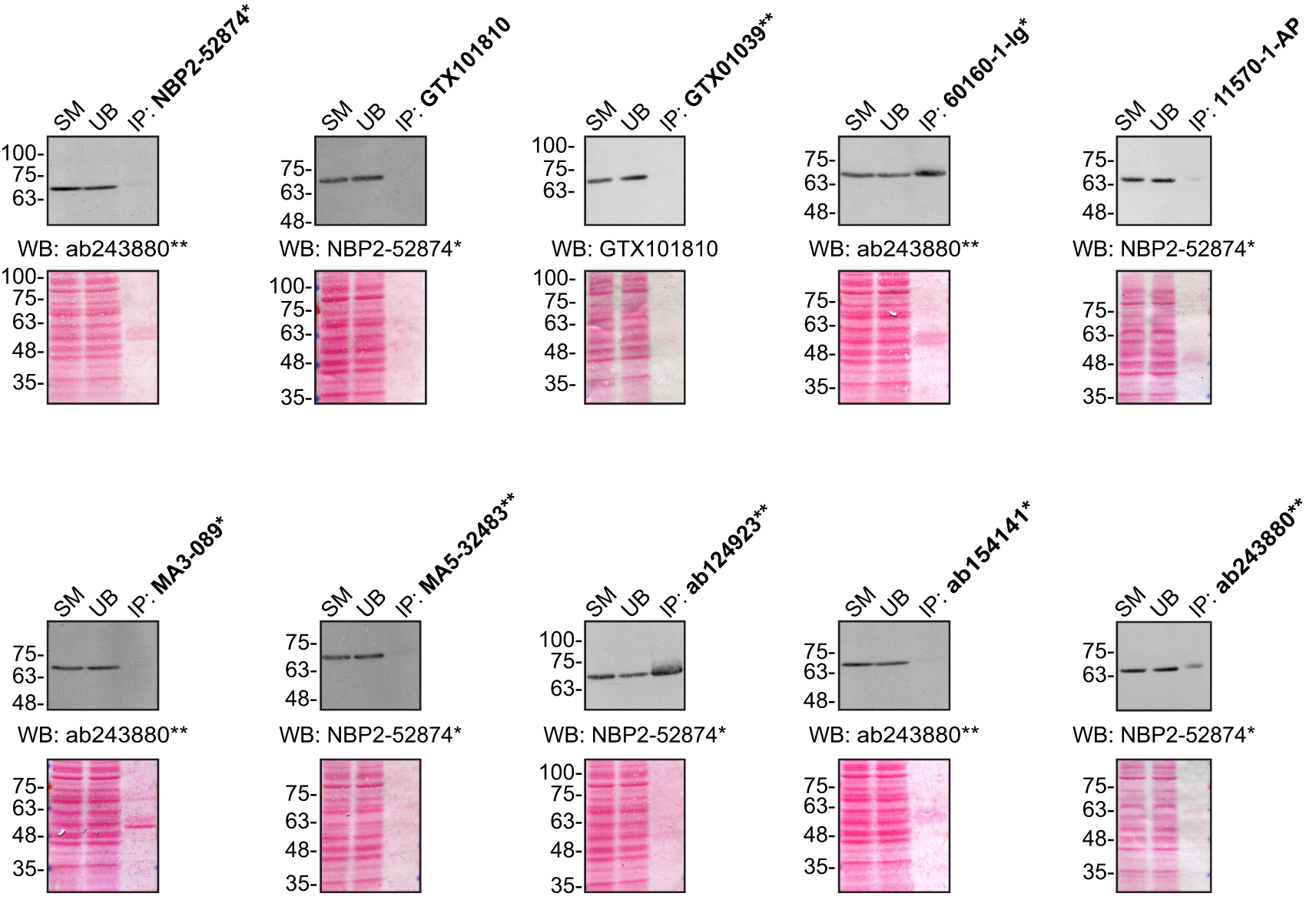
HeLa lysates were prepared, and IP was performed using 1.0 μg of the indicated FUS antibodies pre-coupled to protein G or protein A Sepharose beads. Samples were washed and processed for western blot with the indicated FUS antibody. For western blot, NBP2-52874* and ab243880** were used at a dilution of 1/2000. The Ponceau stained transfers of each blot are shown for similar reasons as in Figure 1. SM=10% starting material; UB=10% unbound fraction; IP=immunoprecipitated. *Monoclonal antibody; **Recombinant antibody.
For immunofluorescence, as described previously, antibodies were screened using a mosaic strategy.23 In brief, we plated WT and KO cells together in the same well and imaged both cell types in the same field of view to reduce staining, imaging and image analysis bias (Figure 3).

HeLa WT and FUS KO cells were labelled with a green or a far-red fluorescent dye, respectively. WT and KO cells were mixed and plated to a 1:1 ratio on coverslips. Cells were stained with the indicated FUS antibodies and with the corresponding Alexa-fluor 555 coupled secondary antibody. Acquisition of the green (identification of WT cells), red (antibody staining) and far-red (identification of KO cells) channels was performed. Representative images of the red (grayscale) channels are shown. WT and KO cells are outlined with yellow and magenta dashed line, respectively. Antibody dilutions were chosen according to the recommendations of the antibody supplier. Exceptions were given for antibodies GTX101810, GTX01039*, 60160-1-Ig*, 11570-1-AP, MA5-32483** and ab124923**, which were titrated as the signals were too weak when following the supplier’s recommendations. Antibody dilution used: NBP2-52874* at 1/1000; GTX101810 at 1/700; GTX01039* at 1/1000; 60160-1-Ig* at 1/2000; 11570-1-AP at 1/1000; MA3-089* at 1/1000; MA5-32483** at 1/1000; ab124923** at 1/1000; ab154141* at 1/1000; ab243880** at 1/500. Bars=10 μm. *Monoclonal antibody; **Recombinant antibody.
For flow cytometry, HeLa WT and FUS KO cells were labelled with distinct fluorescent dyes and combined at a 1:1 ratio. Fixation, permeabilization and blocking procedures were performed in the same tube prior to antibody staining to reduce bias. Ten FUS antibodies were then evaluated and fluorescent intensity was evaluated using the Attune NxT flow cytometer. Antibody staining in WT and KO lines were evaluated using FlowJo software, with representative histograms presented (Figure 4).
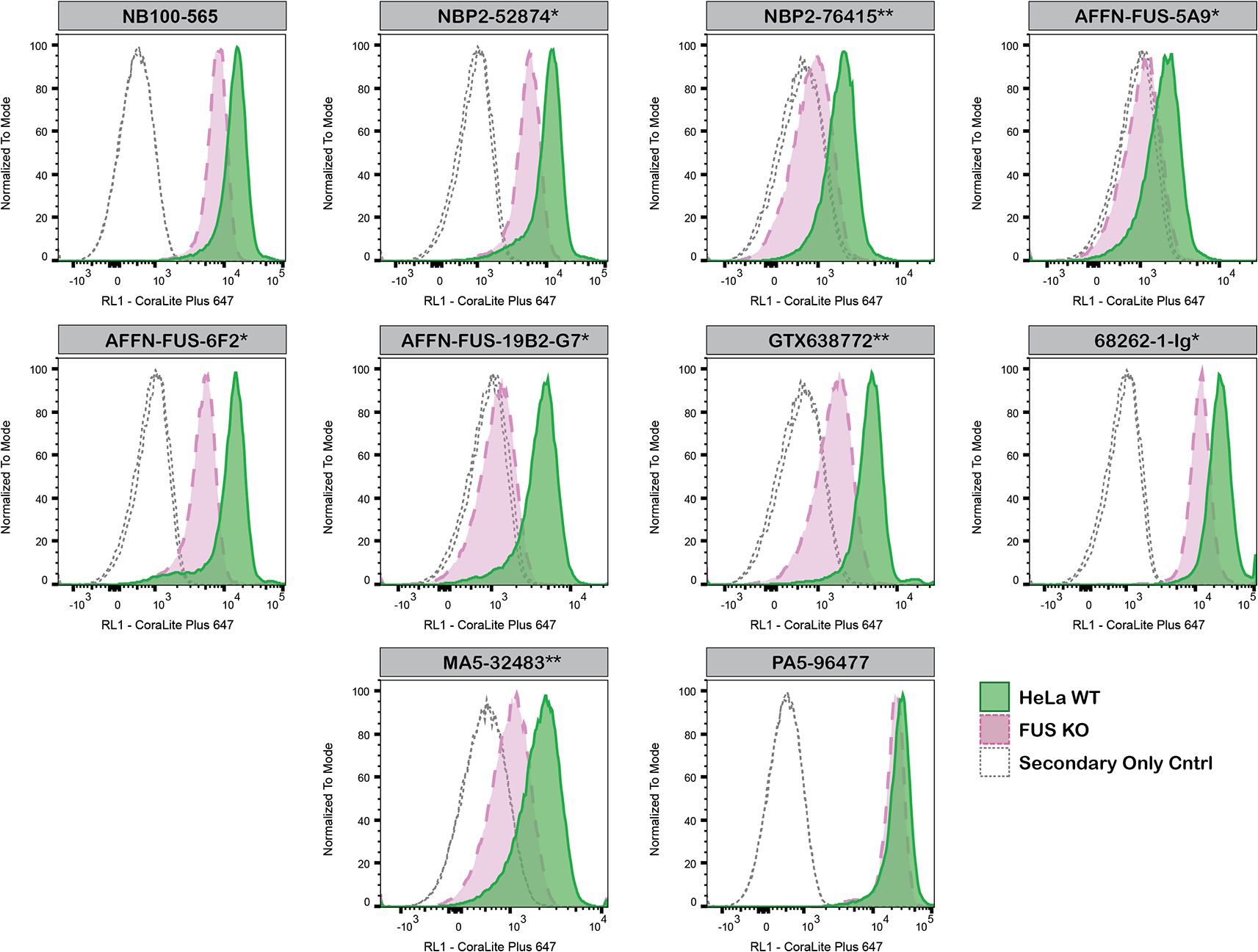
HeLa WT and FUS KO cells were labelled with a green or violet, fluorescent dye, respectively. WT and KO cells were mixed in a 1:1 ratio, fixed in 4% PFA and permeabilized in 0.1% Triton X-100. 400,000 cells were stained with the indicated FUS antibodies and corresponding Multi-rAb CoraLite® Plus 647 secondary antibodies. Antibody staining was quantified using the Attune NxT Flow Cytometer with representative images showing the staining intensity in the KO population (pink histogram, dashed line) compared to the WT cells (green histogram, solid line). Histograms with dotted lines represent secondary antibody-only controls in both WT and KO cells. Antibody concentrations used: NB100-565 at 0.2 μg/mL; NBP2-52874* at 0.2 μg/mL; NBP2-76415** at 1 μg/mL; AFFN-FUS-5A9* at 0.2 μg/mL; AFFN-FUS-6F2* at 0.2 μg/mL; AFFN-FUS-19B2-G7* at 0.2 μg/mL; GTX638772** at 1 μg/mL; 68262-1-Ig* at 0.2 μg/mL; MA5-32483** at 0.2 μg/mL; PA5-96477 at 0.2 μg/mL. *Monoclonal antibody; **Recombinant antibody.
In conclusion, we have screened FUS commercial antibodies by western blot, immunoprecipitation, immunofluorescence and flow cytometry by comparing the signal produced by the antibodies in human HeLa WT and FUS KO cells. To assist users in assessing antibody performance, Table 3 outlines the possible scenarios in which antibodies may perfrom in all four applications. Several high-quality and renewable antibodies that successfully detect FUS were identified in all applications. Researchers who wish to study FUS in a different species are encouraged to select high-quality antibodies, based on the results in this study, and investigate the predicted species reactivity of the manufacturer before extending their research.
The underlying data can be found on Zenodo, an open-access repository for which all YCharOS antibody characterization data can be found.24,25
The standardized protocols used to carry out this KO cell line-based antibody characterization platform was established and approved by a collaborative group of academics, industry researchers and antibody manufacturers. The detailed materials and step-by-step protocols used to characterize antibodies in western blot, immunoprecipitation and immunofluorescence are openly available on Protocol Exchange (10.21203/rs.3.pex-2607/v1).14
All FUS antibodies are listed in Table 2, together with their corresponding Research Resource Identifiers, or RRID, to ensure the antibodies are cited properly.26 Peroxidase-conjugated goat anti-rabbit and anti-mouse antibodies are from Thermo Fisher Scientific (cat. number 65-6120 and 62-6520). Alexa-555-conjugated goat anti-rabbit and anti-mouse secondary antibodies are from Thermo Fisher Scientific (cat. number A21429 and A21424).
| Company | Catalog number | Lot number (used in Wb, IP and IF) | Lot number (used in FC) | RRID (Antibody Registry) | Clonality | Clone ID | Host | Concentration (μg/μL) | Vendors recommended applications |
|---|---|---|---|---|---|---|---|---|---|
| Bio Techne (Novus Biologicals) | NB100-565 | - | A4 | AB_2105207 | Polyclonal | - | Rabbit | 0.2 | Wb, IP, IF |
| Bio Techne (Novus Biologicals) | NBP2-52874* | MAB-03520 | MAB-03693 | AB_2885157 | monoclonal | CL0190 | mouse | 1 | Wb, IF |
| Bio Techne (Novus Biologicals) | NBP2-76415** | - | 2 | AB_3403555 | recombinant-mono | BLR023E | rabbit | 1 | Wb, IP, IF |
| DSHB | AFFN-FUS-5A9* | - | 4/6/17 | AB_2617599 | monoclonal | - | mouse | 0.2 | - |
| DSHB | AFFN-FUS-6F2* | - | 1/26/23 | AB_2617600 | monoclonal | - | mouse | 0.2 | - |
| DSHB | AFFN-FUS-19B2-G7* | - | 4/1/21 | AB_2721973 | monoclonal | - | mouse | 2 | - |
| GeneTex | GTX101810 | 40366 | - | AB_2036972 | polyclonal | - | rabbit | 0.7 | Wb |
| GeneTex | GTX01039* | 822100287 | - | AB_2888934 | monoclonal | JJ09-31 | rabbit | 1 | Wb, IF |
| GeneTex | GTX638772** | - | 45159 | AB_3403041 | recombinant-mono | HL2454 | rabbit | 1 | Wb, IF |
| Proteintech | 60160-1-Ig* | 10017695 | - | AB_10666169 | monoclonal | 3A10B5 | mouse | 2.36 | Wb, IP, IF |
| Proteintech | 11570-1-AP | 86256 | - | AB_2247082 | polyclonal | - | rabbit | 0.9 | Wb, IP, IF |
| Proteintech | 68262-1-Ig* | - | 10029748 | AB_2935347 | monoclonal | 1B4F8 | mouse | 0.2 | Wb, IP, FC |
| Thermo Fisher Scientific | MA3-089* | VB301448 | - | AB_2633334 | monoclonal | 1FU-1D2 | mouse | n/a | Wb, IF |
| Thermo Fisher Scientific | MA5-32483** | VL3152611 | YJ4089243A | AB_2809760 | recombinant-mono | JJ09-31 | rabbit | 1 | Wb, IF |
| Thermo Fisher Scientific | PA5-96477 | - | YJ4090053A | AB_2808279 | polyclonal | - | rabbit | 0.2 | Wb, IP, IF |
| Abcam | ab124923** | GR85761-9 | - | AB_10972861 | recombinant-mono | EPR5812 | rabbit | 0.15 | Wb, IF |
| Abcam | ab154141* | GR3368481-1 | - | AB_2885092 | monoclonal | CL0190 | mouse | 1 | Wb, IF |
| Abcam | ab243880** | GR3376392-2 | - | AB_2885123 | recombinant-mono | BLR023E | rabbit | n/a | Wb, IP, IF |
The HeLa FUS KO clone was generated with low passage cells using an open-access protocol available on Zenodo.org: https://zenodo.org/record/3875777#.ZA-Rxi-96Rv. Two guide RNAs were used to introduce a STOP codon in the FUS gene (sequence guide 1: AGGGAGUCACAAAAGCCACC, sequence guide 2: GGUACGGUGGUGUUGAUGUC).
Both HeLa WT and FUS KO cell lines used are listed in Table 1, together with their corresponding RRID, to ensure the cell lines are cited properly.27 Cells were cultured in DMEM high-glucose (GE Healthcare cat. number SH30081.01) containing 10% fetal bovine serum (Wisent, cat. number 080450), 2 mM L-glutamate (Wisent cat. number 609065), 100 IU penicillin and 100 μg/mL streptomycin (Wisent cat. number 450201).
Western blots were performed as described in our standard operating procedure. HeLa WT and FUS KO were collected in RIPA buffer (50 mM Tris pH 8.0, 150 mM NaCl, 1.0 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate, 0.1% SDS) supplemented with 1× protease inhibitor cocktail mix (MilliporeSigma, cat. number P8340). Lysates were sonicated briefly and incubated for 30 min on ice. Lysates were spun at ~110,000 × g for 15 min at 4°C and equal protein aliquots of the supernatants were analyzed by SDS-PAGE and Western Blot. BLUelf prestained protein ladder from GeneDireX (cat. number PM008-0500) was used.
Western blots were performed with large 5-16% polyacrylamide gels and transferred on nitrocellulose membranes. Proteins on the blots were visualized with Ponceau S staining (Thermo Fisher Scientific, cat. number BP103-10) which is scanned to show together with individual western blot. Blots were blocked with 5% milk for 1 hr, and antibodies were incubated overnight at 4°C with 5% bovine serum albumin (BSA) (Wisent, cat. number 800-095) in TBS with 0.1% Tween 20 (TBST) (Cell Signaling Technology, cat. number 9997). Following three washes with TBST, the peroxidase conjugated secondary antibody was incubated at a dilution of ~0.2 μg/mL in TBST with 5% milk for 1 hr at room temperature followed by three washes with TBST. Membranes were incubated with Pierce ECL (Thermo Fisher Scientific, cat. number 32106) prior to detection with the HyBlot CL autoradiography films (Denville, cat. number 1159T41).
Immunoprecipitation was performed as described in our standard operating procedure. Antibody-bead conjugates were prepared by adding 1.0 μg of antibody to 500 μL of phosphate-buffered saline (PBS) (Wisent, cat. number 311-010-CL) with 0,01% triton X-100 (Thermo Fisher Scientific, cat. number BP151-500) in a 1.5 mL microcentrifuge tube, together with 30 μL of protein A- (for rabbit antibodies) or protein G- (for mouse antibodies) Sepharose beads. Tubes were rocked overnight at 4°C followed by two washes to remove unbound antibodies.
HeLa WT were collected in HEPES buffer (20 mM HEPES, 100 mM sodium chloride, 1 mM EDTA, 1% Triton X-100, pH 7.4) supplemented with protease inhibitor. Lysates were rocked 30 min at 4°C and spun at 110,000 × g for 15 min at 4°C. One mL aliquots at 1.0 mg/mL of lysate were incubated with an antibody-bead conjugate for ~2 hours at 4°C. The unbound fractions were collected, and beads were subsequently washed three times with 1.0 mL of HEPES lysis buffer and processed for SDS-PAGE and western blot on a 5-16% polyacrylamide gels.
Immunofluorescence was performed as described in our standard operating procedure.17–20,23 HeLa WT and FUS KO were labelled with a green and a far-red fluorescence dye, respectively. The fluorescent dyes used are from Thermo Fisher Scientific (cat. number C2925 and C34565). WT and KO cells were plated on glass coverslips as a mosaic and incubated for 24 hrs in a cell culture incubator at 37 oC, 5% CO2. Cells were fixed in 4% paraformaldehyde (PFA) (Beantown chemical, cat. number 140770-10 ml) in PBS for 15 min at room temperature and then washed 3 times with PBS. Cells were permeabilized in PBS with 0.1% Triton X-100 for 10 min at room temperature and blocked with PBS with 5% BSA, 5% goat serum (Gibco, cat. number 16210-064) and 0.01% Triton X-100 for 30 min at room temperature. Cells were incubated with IF buffer (PBS, 5% BSA, 0.01% Triton X-100) containing the primary FUS antibodies overnight at 4°C. Cells were then washed 3 × 10 min with IF buffer and incubated with corresponding Alexa Fluor 555-conjugated secondary antibodies in IF buffer at a dilution of 1.0 μg/mL for 1 hr at room temperature with DAPI. Cells were washed 3 × 10 min with IF buffer and once with PBS. Coverslips were mounted on a microscopic slide using fluorescence mounting media (DAKO).
Imaging was performed using a Zeiss LSM 880 laser scanning confocal microscope equipped with a Plan-Apo 40× oil objective (NA = 1.40). Analysis was done using the Zen navigation software (Zeiss). All cell images represent a single focal plane. Figures were assembled with Adobe Photoshop (version 24.1.2) to adjust contrast then assembled with Adobe Illustrator (version 27.3.1).
HeLa WT and FUS KO cells were detached, and five million cells were labelled with CellTracker green or violet fluorescent dyes, respectively (Thermo Fisher Scientific, cat. number C7025 and C10094). WT and KO cells were then centrifuged at 300 × g, for 10 min and resuspended in PBS containing 1% bovine serum albumin (BSA) (Sigma-Aldrich, A9647). Cell populations were then combined at a 1:1 ratio, centrifuged and fixed on ice for 20 min using 800 μL of 4% PFA in PBS (Thermo Fisher Scientific, cat. number J19943.K2). Following fixation, 1.2 mL of 1% BSA in PBS was added to the tube, vortexed and centrifuged at 600 × g for 15 min at 4°C. Cells were then permeabilized in 400 μL PBS with 0.1% Triton X-100 (Thermo Fisher Scientific, J63521.AP) for 10 min at room temperature. Following permeabilization 1mL of 1% BSA PBS was added to the tube, vortexed and centrifuged at 600 × g for 15 min at 4oC. Cells were then blocked in 5% goat serum (Sigma-Aldrich, G6767), 1% BSA in PBS for 30 min on ice. After the blocking step 400,000 cells were aliquoted into individually labelled tubes, centrifuged 600 × g for 15 min at 4°C and incubated in 150 μL of 1% BSA PBS with primary FUS antibodies for 30 min on ice. 500 μL of PBS with 1% BSA was added to each tube, vortexed and centrifuged at 600 × g for 15 min at 4°C. Cells were then incubated with the corresponding Multi-rAb CoraLite® Plus 647 secondary antibodies (0.83 μg/ml) in 150 μL of PBS containing 1% BSA for 30 min on ice. For mouse primary antibodies Multi-rAb CoraLite Plus 647-goat-anti-mouse was used (Proteintech, RGAM005). For rabbit primary antibodies Multi-rAb CoraLite Plus 647-goat-anti-rabbit was used (Proteintech, RGAR005). 500 μl of PBS containing 1% BSA was then added to each tube, vortexed and centrifuged 600 × g 15 min at 4°C. Tubes were then resuspended in 1 ml of 1% BSA in PBS and data was acquired using the Attune NxT flow cytometer. Data was analysed using FlowJo with the following gates. The cell population was gated on FSC-A vs SSC-A, within that gate single cells were selected by FSC-A vs FSC-H and then KO and WT cells were isolated by BL1-A vs VL1-A using a quadrat gate. Quantification of antibody staining was then observed in the RL1-A channel and histograms merged to demonstrate the staining intensity between the two populations compared to the two secondary only controls. The figure was then assembled using Adobe Illustrator 2024.
Inherent limitations are associated with the antibody characterization platform used in this study. Firstly, the YCharOS project focuses on renewable (recombinant and monoclonal) antibodies and does not test all commercially available FUS antibodies. YCharOS partners provide approximately 80% of all renewable antibodies, but some top-cited polyclonal antibodies may not be available through these partners.
Secondly, the YCharOS effort employs a non-biased approach that is agnostic to the protein for which antibodies have been characterized. The aim is to provide objective data on antibody performance without preconceived notions about how antibodies should perform or the molecular weight that should be observed in western blot. As the authors are not experts in FUS only a brief overview of the protein’s function and its relevance in disease is provided. RNA-binding protein experts are responsible for analyzing and interpreting observed banding patterns in western blots and subcellular localization in immunofluorescence.
Thirdly, YCharOS experiments are not performed in replicates primarily due to the use of multiple antibodies targeting various epitopes. Once a specific antibody is identified, it validates the protein expression of the intended target in the selected cell line, confirms the lack of protein expression in the KO cell line and supports conclusions regarding the specificity of the other antibodies. Moreover, the same antibody clones are donated by 2-3 manufacturers (cross-licensed antibodies), effectively serving as replicates and enabling the validation of test reproducibility. All experiments are performed using master mixes, and meticulous attention is paid to sample preparation and experimental execution. In IF, the use of two different concentrations serves to evaluate antibody specificity and can aid in assessing assay reliability. In instances where antibodies yield no signal, a repeat experiment is conducted following titration. Additionally, our independent data is performed subsequently to the antibody manufacturers internal validation process, therefore making our characterization process a repeat.
Lastly, as comprehensive and standardized procedures are respected, any conclusions remain confined to the experimental conditions and cell line used for this study. The use of a single cell type for evaluating antibody performance poses as a limitation, as factors such as target protein abundance significantly impact results.14 Additionally, the use of cancer cell lines containing gene mutations poses a potential challenge, as these mutations may be within the epitope coding sequence or other regions of the gene responsible for the intended target. Such alterations can impact the binding affinity of antibodies. This represents an inherent limitation of any approach that employs cancer cell lines.
Zenodo: Antibody Characterization Report for RNA-binding protein FUS, https://doi.org/10.5281/zenodo.5259944. 24
Zenodo: Dataset for the RNA-binding protein FUS antibody screening study, https://doi.org/10.5281/zenodo.13736595. 25
Data are available under the terms of the Creative Commons Attribution 4.0 International license (CC-BY 4.0).
We would like to thank the NeuroSGC/YCharOS/EDDU collaborative group for their important contribution to the creation of an open scientific ecosystem of antibody manufacturers and knockout cell line suppliers, for the development of community-agreed protocols, and for their shared ideas, resources and collaboration. Members of the group can be found below.
NeuroSGC/YCharOS/EDDU collaborative group: Riham Ayoubi, Thomas M. Durcan, Aled M. Edwards, Carl Laflamme, Peter S. McPherson, Chetan Raina, Kathleen Southern and Zhipeng You.
Thank you to the Institute for Precision Health and Leicester Drug Discovery and Diagnostics (LD3) via the MRC Impact Accelerator Account award (MR/X502777/1) for supporting this study.
An earlier version of this of this article can be found on Zenodo (https://doi.org/10.5281/zenodo.5259945)
| Views | Downloads | |
|---|---|---|
| F1000Research | - | - |
|
PubMed Central
Data from PMC are received and updated monthly.
|
- | - |
Competing Interests: No competing interests were disclosed.
References
1. Yan J, Deng HX, Siddique N, Fecto F, et al.: Frameshift and novel mutations in FUS in familial amyotrophic lateral sclerosis and ALS/dementia.Neurology. 2010; 75 (9): 807-14 PubMed Abstract | Publisher Full TextCompeting Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular biology, neuroscience
Is the rationale for creating the dataset(s) clearly described?
Yes
Are the protocols appropriate and is the work technically sound?
Yes
Are sufficient details of methods and materials provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Is the rationale for creating the dataset(s) clearly described?
Yes
Are the protocols appropriate and is the work technically sound?
Yes
Are sufficient details of methods and materials provided to allow replication by others?
Yes
Are the datasets clearly presented in a useable and accessible format?
Yes
Competing Interests: No competing interests were disclosed.
Reviewer Expertise: Molecular biology, neuroscience
Alongside their report, reviewers assign a status to the article:
| Invited Reviewers | ||
|---|---|---|
| 1 | 2 | |
|
Version 3 (revision) 24 Sep 24 |
read | |
|
Version 2 (revision) 26 Jun 23 |
read | |
|
Version 1 06 Apr 23 |
read | read |
Provide sufficient details of any financial or non-financial competing interests to enable users to assess whether your comments might lead a reasonable person to question your impartiality. Consider the following examples, but note that this is not an exhaustive list:
Sign up for content alerts and receive a weekly or monthly email with all newly published articles
Already registered? Sign in
The email address should be the one you originally registered with F1000.
You registered with F1000 via Google, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Google account password, please click here.
You registered with F1000 via Facebook, so we cannot reset your password.
To sign in, please click here.
If you still need help with your Facebook account password, please click here.
If your email address is registered with us, we will email you instructions to reset your password.
If you think you should have received this email but it has not arrived, please check your spam filters and/or contact for further assistance.
Comments on this article Comments (0)